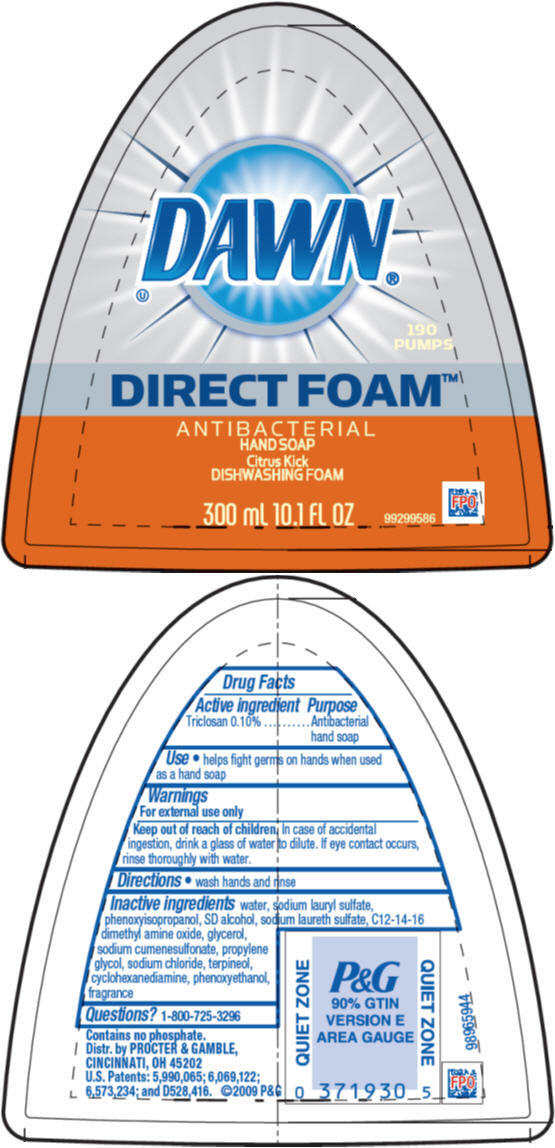
DAWN DIRECT FOAM CITRUS KICK- triclosan soap
Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dawn Direct Foam Citrus Kick AB
| DAWN
DIRECT FOAM CITRUS KICK
triclosan soap |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Company (004238200) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The Procter & Gamble Manufacturing Company | 007130032 | ANALYSIS(37000-610), LABEL(37000-610), MANUFACTURE(37000-610), PACK(37000-610), RELABEL(37000-610), REPACK(37000-610) | |
Revised: 6/2014
Document Id: cdebdb43-d577-4fc3-bf81-22060e65969a
Set id: df66e44d-7242-4669-9ea3-8f572fce89e0
Version: 2
Effective Time: 20140609
Procter & Gamble Manufacturing Company