CINRYZE
-
human c1-esterase inhibitor injection, powder, lyophilized, for solution
ViroPharma Biologics, Inc.
----------
|
|||||||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
CINRYZE™ (C1 Esterase Inhibitor [Human])
Freeze dried powder
1 INDICATIONS AND USAGE
CINRYZE is a C1 esterase inhibitor indicated for routine prophylaxis against angioedema attacks in adolescent and adult patients with Hereditary Angioedema (HAE).
2 DOSAGE AND ADMINISTRATION
For Intravenous Use, Freeze-Dried powder for Reconstitution.
2.1 Routine prophylaxis against HAE Attacks
- A dose of 1,000 Units CINRYZE can be administered every 3 or 4 days for routine prophylaxis against angioedema attacks in HAE patients.
- CINRYZE is administered at an injection rate of 1 mL per minute.
| Indication | Dose | Initial Infusion rate | Maintenance infusion rate (if tolerated) |
| Routine prophylaxis against HAE attacks | 1,000 units Intravenous every 3 or 4 days | 1 mL/min (10 min) | 1 mL/min (10 min) |
2.2 Instructions for Use
The procedures below are provided as general guidelines for the reconstitution and administration of CINRYZE.
Always work on a clean surface and wash your hands before performing the following procedures.
Reconstitution, product administration, and handling of the administration set and needles must be done with caution. Percutaneous puncture with a needle contaminated with blood can transmit infectious viruses including HIV (AIDS) and hepatitis. Obtain immediate medical attention if injury occurs. Place needles in a sharps container after single use. Discard all equipment, including any reconstituted CINRYZE in an appropriate container.
2.3 Preparation and Handling
- Prior to reconstitution, CINRYZE should be protected from light.
- CINRYZE should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted solution should be colorless to slightly blue, and free from visible particles. Do not use if turbid or discolored.
- The CINRYZE vial is for single use only. CINRYZE contains no preservative. Any vial that has been entered should be used promptly. Partially used vials should be discarded in accordance with biohazard procedures.
- Do not mix CINRYZE with other materials.
- Do not use if frozen.
- Do not use after expiration date.
Reconstitution:
Two vials of reconstituted CINRYZE are combined for a single dose. Sterile Water for Injection, USP, is required and not supplied with CINRYZE.
- Aseptic technique should be used during the reconstitution procedure.
- Bring the CINRYZE (powder) and Sterile Water for Injection, USP (diluent) (not supplied) to room temperature if refrigerated.
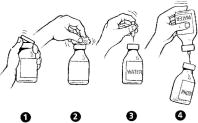
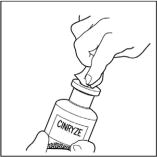
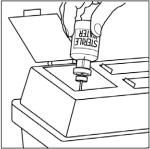
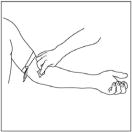
- Remove caps from the CINRYZE and diluent vials (Step 1).
- Cleanse stoppers with germicidal solution, and allow them to dry prior to use. (Step 2)
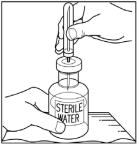
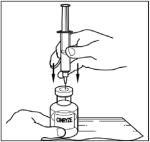
- Remove protective covering from one end of the double-ended transfer needle and insert exposed needle through the center of the diluent vial stopper (Step 3).
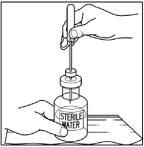
- Remove protective covering from the other end of the double-ended transfer needle. Invert diluent vial containing 5 mL Sterile Water for Injection, USP, over the upright and slightly angled CINRYZE vial allowing (Step 4); then rapidly insert the free end of the needle through the CINRYZE vial stopper at its center. The vacuum in the vial will draw in the diluent. If there is no vacuum in the vial, do not use the product.
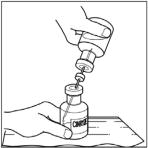
- Disconnect the two vials by removing the needle from the CINRYZE vial stopper and discard the diluent vial, along with the transfer needle directly into the sharps container. Gently swirl the CINRYZE vial until all powder is dissolved. Be sure that CINRYZE is completely dissolved.
One vial of reconstituted CINRYZE contains 5 mL of C1 esterase inhibitor at a concentration of 100 Units/mL. Reconstitute two vials of CINRYZE for one dose.
2.4 Administration:
Two vials of reconstituted CINRYZE are combined for a single dose.
- Use Aseptic Technique.
- After reconstitution, the solution is colorless to slightly blue and clear. Do not use the product if the solution is turbid or discolored.
- CINRYZE must be administered at room temperature within 3 hours after reconstitution.
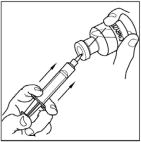
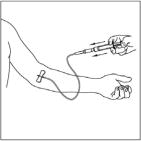
- Attach the filter needle to a sterile, disposable syringe and draw back the plunger to admit air into the syringe.
- Insert the filter needle into the vial of reconstituted CINRYZE.
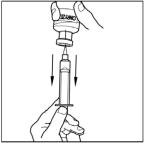
- Inject air into the vial and then withdraw the reconstituted CINRYZE into the syringe. This should be repeated with a second vial of CINRYZE to make the complete dose.
- Remove and discard the filter needle in a hard-walled sharps container for proper disposal. Filter needles are intended to filter the contents of a single dose (2 vials) of CINRYZE only.
- Attach a suitable needle or infusion set with winged adapter, and inject intravenously. As a guideline, administer 1,000 Units ( reconstituted in 10ml) of CINRYZE by intravenous injection at a rate of 1 mL per minute over 10 minutes (see Clinical Studies, Section 14)
- Dispose of all unused solution, the empty vial(s), and the used needles and syringes in an appropriate container for throwing away waste that might hurt others if not handled properly.
3. DOSAGE FORMS AND STRENGTHS
- CINRYZE is a lyophilized preparation available in a single-use vial that contains 500 Units (U) human C1 esterase inhibitor.
- Each vial must be reconstituted with 5 mL Sterile Water for Injection, USP (diluent) (not supplied).
- Two reconstituted vials must be used to make a single, 1,000 Units, dose.
4 CONTRAINDICATIONS
CINRYZE is contraindicated in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis to the product.
5 WARNINGS AND PRECAUTIONS
5.1 Sensitivity
Severe hypersensitivity reactions may occur. The signs and symptoms of hypersensitivity reactions may include the appearance of hives, urticaria, tightness of the chest, wheezing, hypotension and/or anaphylaxis experienced during or after injection of CINRYZE.
Because hypersensitivity reactions may have symptoms similar to HAE attacks, treatment methods should be carefully considered.
In case of hypersensitivity, CINRYZE infusion should be discontinued and appropriate treatment instituted. Epinephrine should be immediately available for treatment of acute severe hypersensitivity reaction. (See Patient Counseling Information [17])
5.2 Thrombotic Events
Thrombotic events have been reported in association with C1 esterase inhibitor products when used off-label at high doses.6 Animal studies have supported a concern about the risk of thrombosis from intravenous administration of C1 esterase inhibitor products.7 (see Sections 10 OVERDOSAGE and 13.2 Animal Toxicology and/or Pharmacology)
5.3 Transmissible Infectious Agents
Because CINRYZE is made from human blood, it may carry a risk of transmitting infectious agents, e.g. viruses, and, theoretically, the Creutzfeldt-Jakob (CJD) agent [5.3, 11]. ALL infections thought by a physician possibly to have been transmitted by CINRYZE should be reported by the physician or other healthcare provider to ViroPharma Biologics, Inc [(877) 945-1000]. The physician should discuss the risks and benefits of this product with the patient, before prescribing or administering it to the patient (See Patient Counseling Information [17]).
6 ADVERSE REACTIONS
6.1 Adverse Reaction Overview
The most serious adverse events observed in clinical studies of CINRYZE have been death due to non-catheter related foreign body embolus, pre-eclampsia resulting in emergency C-section, stroke, and exacerbation of HAE attacks, none of which have been considered drug related.
The most common drug related adverse reactions observed at a rate ≥ 5% were upper respiratory tract infections, sinusitis, rash, and headache.
6.2 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Routine Prophylaxis
Twenty-four subjects were evaluated in study LEVP2005-1/B for routine prophylaxis.
There were no treatment-emergent serious adverse reactions in study LEVP2005-1/B.
Adverse reactions in trial LEVP2005-1/B that occurred in at least two subjects during CINRYZE prophylaxis, irrespective of the causality assessment, are given in the following table:
| Adverse Reaction | Number of Adverse Events | Number of Subjects (N = 24) |
|---|---|---|
| Sinusitis | 8 | 5 |
| Rash | 7 | 5 |
| Headache | 4 | 4 |
| Upper respiratory tract infection | 3 | 3 |
| Viral upper respiratory tract infection | 5 | 3 |
| Bronchitis | 2 | 2 |
| Limb injury | 2 | 2 |
| Back pain | 2 | 2 |
| Pain in extremity | 2 | 2 |
| Pruritus | 2 | 2 |
More than 9000 doses of CINRYZE have been administered to over 180 patients in all controlled and open label clinical studies. All patients were evaluated and found negative for seroconversion to parvovirus B19, Hepatitis B, Hepatitis C and HIV.
7 DRUG INTERACTIONS
No drug interaction studies have been conducted.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. No animal data are available. No adequate and well-controlled studies were conducted in pregnant women. It is not known whether CINRYZE can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. CINRYZE should be given to a pregnant woman only if clearly needed.
8.2 Labor and Delivery
The safety and effectiveness of CINRYZE administration prior to or during labor and delivery have not been established. Use only if clearly needed.
8.3 Nursing Mothers
It is not known whether CINRYZE is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when CINRYZE is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of CINRYZE have not been established in neonates, infants, or children. Three of the 24 subjects in Study LEVP2005-1/B were under the age of 18 years (9, 14, and 16 years of age).
8.5 Geriatric Use
The clinical study LEVP2005-1/B did not include sufficient numbers of subject 65 years of age and older to determine whether they respond differently from younger subjects.
10 OVERDOSAGE
The maximum dose administered in clinical studies was 4000 Units given over approximately 5 hours (an average dose of 57 Units/kg) and 9000 Units given over a 7 day period. There have been no overdosages of CINRYZE reported during clinical studies.
In vitro and in vivo animal thrombogenicity studies with CINRYZE showed a potential for clot formation when CINRYZE was administered at doses 14 times the recommended clinical dose (greater than 200U/kg). Thrombotic events have been reported in association with C1 esterase inhibitor products when used off-label at high doses.6 Animal studies have supported a concern about the risk of thrombosis from intravenous administration of C1 esterase inhibitor products.7 (see Section 13.2 Animal Toxicology and/or Pharmacology and Section 5.2 Thrombotic events in WARNINGS AND PRECAUTIONS).
11 DESCRIPTION
CINRYZE (C1 esterase inhibitor [Human]) is a sterile, stable, lyophilized preparation of C1 esterase inhibitor derived from human plasma. CINRYZE is manufactured from human plasma purified by a combination of filtration and chromatographic procedures. The specific activity of CINRYZE is 4.0 – 9.0 units/mg protein. The purity is ≥ 90% human C1 esterase inhibitor. Following reconstitution with 5 mL of Sterile Water for Injection, USP, each vial contains approximately 500 units of functionally active C1 esterase inhibitor, pH 6.6 - 7.4, and an osmolality between 200 – 400 mosmol/kg. One unit (U) of CINRYZE corresponds to the mean quantity of C1 esterase inhibitor present in 1 ml of normal fresh plasma.
CINRYZE, when reconstituted with 5 mL of Sterile Water for Injection contains the following excipients: 4.1 mg/mL sodium chloride, 21 mg/mL sucrose, 2.6 mg/mL trisodium citrate, 2.0 mg/mL L-Valine, 1.2 mg/mL L-Alanine, and 4.5 mg/mL L-Threonine.
The following manufacturing steps are designed to reduce the risk of viral transmission:
- Screening donors at U.S. licensed blood collection centers to rule out infection with Human Immunodeficiency Virus (HIV-1/HIV-2), Hepatitis B Virus, or Hepatitis C Virus.
- Testing plasma pools by in-process NAT for parvovirus B19 via minipool testing and the limit of B19 in the manufacturing pool is set not to exceed 104 IU of B19 DNA per mL.
- Use of two independent viral reduction steps in the manufacture of CINRYZE: pasteurization (heat treatment at 60°C for 10 hours in solution with stabilizers) and nanofiltration through two sequential 15 nm Planova filters.
These viral reduction steps, along with a step in the manufacturing process, PEG precipitation, have been validated in a series of in vitro experiments for their capacity to inactivate/remove a wide range of viruses of diverse physicochemical characteristics including: Human Immunodeficiency Virus (HIV), Hepatitis A Virus (HAV), and the following model viruses: Bovine Viral Diarrhea Virus (BVDV) as a model virus for HCV, Canine Parvovirus (CPV) as a model virus for Parvovirus B19, Pseudorabies Virus (PRV) as a model virus for large enveloped DNA viruses (e.g. herpes virus). Total mean log10 reductions are shown in Table 3.
| Process step | Log10 Virus Reduction | ||||
| Enveloped viruses | Non-enveloped viruses | ||||
| HIV | BVDV | PRV | HAV | CPV | |
| PEG precipitation | 5.1 ± 0.2 | 4.5 ± 0.3 | 6.0 ± 0.3 | 2.8 ± 0.2 | 4.2 ± 0.2 |
| Pasteurization | > 6.1 ± 0.2 | > 6.7 ± 0.3 | > 6.7 ± 0.2 | 2.8 ± 0.3 | 0.1 ± 0.3 |
| Nanofiltration | > 5.6 ± 0.2 | > 5.5 ± 0.2 | > 6.4 ± 0.3 | > 4.9 ± 0.2 | > 4.5 ± 0.3 |
| Total reduction | > 16.8 | > 16.7 | > 19.1 | > 10.5 | > 8.7 |
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
C1 inhibitor is a normal constituent of human blood and is one of the serine proteinase inhibitors (serpins). The primary function of C1 inhibitor is to regulate the activation of the complement and intrinsic coagulation (contact system) pathway. C1 inhibitor also regulates the fibrinolytic system. Regulation of these systems is performed through the formation of complexes between the proteinase and the inhibitor, resulting in inactivation of both and consumption of the C1 inhibitor.
HAE patients have low levels of endogenous or functional C1 inhibitor. Although the events that induce attacks of angioedema in HAE patients are not well defined, it is thought by some that increased vascular permeability and the clinical manifestation of HAE attacks are primarily mediated through contact system activation. Suppression of contact system activation by C1 inhibitor through the inactivation of plasma kallikrein and factor XIIa is thought to modulate this vascular permeability by preventing the generation of bradykinin6. Administration of CINRYZE increases plasma levels of C1 inhibitor activity.
12.2 Pharmacodynamics
In clinical studies, the intravenous administration of CINRYZE demonstrated an increase in plasma levels of C1 inhibitor within approximately one hour or less of administration.
Biological activity of CINRYZE was shown in 35 subjects by the subsequent increase in plasma C4 levels from an average of C4 8.1 mg/mL at baseline to C4 8.6 mg/mL 12 hours after infusion of CINRYZE.
12.3 Pharmacokinetics
A randomized, parallel group, open label pharmacokinetics (PK) study of CINRYZE was performed in patients with non-symptomatic hereditary angioedema (HAE). The patients received either a single dose of 1,000 units or 1,000 units followed by a second 1,000 units 60 minutes later. The PK results for functional C1 inhibitor are presented the following table:
|
Numbers in parenthesis are number of subjects evaluated |
||
|
Single dose = 1,000 units |
||
|
Double dose = 1,000 units followed by a second 1,000 units 60 minutes later |
||
|
* One unit is equal to the mean C1 inhibitor concentration of 1 mL of normal human plasma |
||
| Parameters | Single Dose | Double Dose |
| Cbaseline (units/mL) | 0.31 ± 0.20 (n = 12) | 0.33 ± 0.20 (n = 12) |
| Cmax (units/mL) | 0.68 ± 0.08 (n = 12) | 0.85 ± 0.12 (n = 13) |
| Tmax (hrs) | 3.9 ± 7.3 (n = 12) | 2.7 ± 1.9 (n = 13) |
| AUC(0-t) (units*hr/mL) | 74.5 ± 30.3 (n = 12) | 95.9 ± 19.6 (n = 13) |
| CL (mL/min) | 0.85 ± 1.07 (n = 7) | 1.17 ± 0.78 (n = 9) |
| Half-life (hours) | 56 ± 36 (n = 7) | 62 ± 38 (n = 9) |
The maximum plasma concentrations (Cmax) and area under the plasma concentration-time curve (AUC) increased from the single to double dose, although the increase was not dose proportional. The mean half-lives of CINRYZE were 56 hours (range 11 to 108 hours) for a single dose and 62 hours (range 16 to 152 hours) for the double dose.
Studies have not been conducted to evaluate the PK of CINRYZE in special patient populations identified by gender, race, age (pediatric or geriatric), or the presence of renal or hepatic impairment.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been completed to evaluate the effects of CINRYZE on carcinogenesis, mutagenesis, and impairment of fertility.
13.2 Animal Toxicology and/or Pharmacology
Acute toxicity of CINRYZE was studied in a combined acute toxicity and 7-day repeat dose/ dose range finding (DRF) study in Sprague Dawley rats. Repeat dose toxicity was studied in a 7-day repeat dose follow up to the acute dose study. The acute and repeated dose toxicity study were performed with intravenous administration of CINRYZE at dose levels of 1, 7 and 28 times normal dose. No signs of toxicity were observed in the single dose study. In the repeated dose study, no signs of toxicity were observed in the two lower doses. Repeat dosing in the rat resulted in a robust neutralizing antibody response between days 1 and 14. Therefore, toxicity in animals dosed repeatedly is difficult to interpret.
In vitro and in vivo thrombogenicity studies showed a potential for clot formation when CINRYZE was administered at doses 14 times the recommended clinical dose (greater than 200U/kg).
14 CLINICAL STUDIES
14.1 Routine Prophylaxis Trial LEVP2005-1/B
The safety and efficacy of CINRYZE prophylaxis therapy to reduce the incidence, severity, and duration of HAE attacks was demonstrated in a single randomized, double blind, placebo controlled multi-center cross-over study of 24 patients. Patients were screened to confirm a diagnosis of HAE and a history of at least two HAE attacks per month. 24 patients (mean age 38.1 years with a range of 9 to 73 years) were randomized to one of two treatment groups: either CINRYZE prophylaxis for 12 weeks followed by 12 weeks of placebo prophylaxis; or randomized to placebo prophylaxis for 12 weeks followed by 12 weeks of CINRYZE prophylaxis. Two subjects dropped out (one in each arm); 22 patients crossed over into period 2 and were included in the efficacy analysis. Patients were given blinded injections (CINRYZE or placebo) every 3 to 4 days, approximately 2 times per week. Patients recorded all angioedema symptoms daily. An attack was defined as the subject-reported indication of swelling at any location following a report of no swelling on the previous day.
The efficacy determination was based on the number of attacks during the 12 week period while receiving CINRYZE as compared to the number of attacks during the placebo treatment period. The effectiveness of C1 esterase inhibitor prophylaxis in reducing the number of HAE attacks was variable among the subjects as shown in table 5:
| Subject | Percent Reduction in Attack Frequency |
|---|---|
| 1 | 100% |
| 2 | 100% |
| 3 | 100% |
| 4 | 100% |
| 5 | 90% |
| 6 | 88% |
| 7 | 84% |
| 8 | 83% |
| 9 | 78% |
| 10 | 76% |
| 11 | 60% |
| 12 | 47% |
| 13 | 43% |
| 14 | 43% |
| 15 | 32% |
| 16 | 31% |
| 17 | 25% |
| 18 | 21% |
| 19 | 10% |
| 20 | 1% |
| 21 | -8% |
| 22 | -85% |
| Statistic | CINRYZE N=22 | Placebo N=22 | |
| Number of Attacks | Mean | 6.1 | 12.7 |
| SD | 5.4 | 4.8 | |
| Median | 6 | 13.5 | |
| Min | 0 | 6 | |
| Max | 17 | 22 | |
| GEE Analysis Results | |||
| Effect Assessed | p-value | ||
| Treatment Effect | <0.0001 | ||
| Sequence Effect | 0.3347 | ||
| Period Effect | 0.3494 | ||
Patients treated with CINRYZE had a 66% reduction in days of swelling (p<0.0001), and decreases in the average severity of attacks (p=0.0006) and the average duration of attacks (p=0.0023), as shown in table 7.
| CINRYZE
N=22 | Placebo
N=22 | Treatment Effect
p-value |
|
|---|---|---|---|
| Mean Severity of
HAE Attacks (Score from 1 to 3) (SD) | 1.3(0.85) | 1.9 (0.36) | 0.0006 |
| Mean Duration of
HAE Attacks (Days) (SD) | 2.1 (1.13) | 3.4 (1.4) | 0.0023 |
| Days of Swelling
(SD) | 10.1 (10.73) | 29.6 (16.9) | <0.0001 |
15 REFERENCES
- Zuraw, B.2005. Current and future therapy for hereditary angioedema. Clinical Immunology. 114: 10– 16.
- Agostoni A, Aygoren-Pursun E, Binkley KE, et al. 2004. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 114(3 Suppl):S51-131.
- Zwaan, C., Kleine, A., J. H. C. Diris, J., et al., 2002. Continuous 48-h C1-inhibitor treatment, following reperfusion therapy, in patients with acute myocardial infarction. European Heart Journal. 23:1670–1677.
- Caliezi C, Wuillemin WA, Zeerleder S, Redondo M, Eisele B, Hack CE. C1-Esterase Inhibitor: An Anti-Inflammatory Agent and Its Potential Use in the Treatment of Diseases Other Than Hereditary Angioedema. Pharmacol Rev. 2000; 52:91-112
- Davis AE, The pathophysiology of hereditary angioedema. Clin Immunol. 2005; 114:3-9.
- Arzneimittelkommission der Deutschen Aertzteschaft. Schwerwiegende Thrombenbildung nach Berinert HS. Dtsch Aerztebl. 2000; 97:B-864
- Horstick, G et al, 2002. Circulation 104:3125-3131
16 HOW SUPPLIED/STORAGE AND HANDLING
- CINRYZE is available in single-use vials that contain 500 Units per vial.
- CINRYZE is supplied as a single glass vial of CINRYZE powder to be reconstituted with 5 mL Sterile Water for Injection, USP (Not supplied).
- CINRYZE, packaged for sale, is stable for 1 year when stored at 2°C–25°C (36°F-77°F).
- Do not freeze.
- Store the vial in the original carton to protect it from light.
- The reconstituted solution must be used within 3 hours of reconstitution.
- Do not use beyond the expiration date on the CINRYZE vial.
17 PATIENT COUNSELING INFORMATION
17.1 Allergic-type Hypersensitivity Reactions
Allergic-type hypersensitivity reactions are possible [5.1]. Inform patients of the early signs of hypersensitivity reactions [including hives (itchy white elevated patches), tightness of the chest, wheezing, hypotension] and anaphylaxis. Advise patients to discontinue use of CINRYZE and contact their physicians if these symptoms occur.
17.2 Pregnancy
Advise female patients to notify their physician if they become pregnant or intend to become pregnant during their routine prevention with CINRYZE.
17.3 Nursing
Advise patients to notify their physician if they are breastfeeding or plan to breastfeed.
17.4 Usage While Traveling
Based on their current regimen, advise patients to bring an adequate supply of CINRYZE for routine prevention when traveling. Advise patients to consult with their healthcare professional prior to travel.
17.5 Transmissible Infectious Agents
Advise patient that, because CINRYZE is made from human blood, it may carry a risk of transmitting infectious agents, e.g. viruses, and , theoretically, the Creutzfeldt-Jakob (CJD) agent [5.3, 11]. The risk of transmitting disease has been reduced, but not eliminated, by carefully selecting blood donors, testing donors for infections, and inactivating or removing most viruses during the manufacturing process. Inform patients of the risks and benefits of CINRYZE before prescribing or administering to the patient.
17.6 FDA-Approved Patient Labeling
Information for the Patient
CINRYZE™ (SIN-rise)
(C1 Esterase Inhibitor [Human])
This leaflet summarizes important information about CINRYZE. Please read it carefully before using CINRYZE and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about CINRYZE. If you have any questions after reading this, ask your healthcare provider.
Do not attempt to self-administer unless you have been taught how by your healthcare provider.
What is CINRYZE?
CINRYZE is an injectable medicine that is used to help prevent swelling and /or painful attacks in teenagers and adults with Hereditary Angioedema (HAE). HAE is caused by the decreased functioning of a protein called C1 esterase inhibitor, that is present in your blood and helps control inflammation (swelling) and parts of the immune system. CINRYZE contains C1 esterase inhibitor. Before you can inject CINRYZE into your vein (intravenous injection), you must dissolve the CINRYZE powder using Sterile Water for Injection, USP. You can get supplies, including Sterile Water for Injection, USP from your pharmacist.
Who should not use CINRYZE?
You should not use CINRYZE if you have had life-threatening immediate hypersensitivity reactions, including anaphylaxis to the product.
What should I tell my healthcare provider before using CINRYZE?
Tell your healthcare provider about all of your medical conditions, including if you
- are pregnant or planning to become pregnant. It is not known if CINRYZE can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if CINRYZE passes into your milk and if it can harm your baby.
- have a history of blood clotting problems. Very high doses of C1 esterase inhibitor could increase the risk of blood clots.
Tell your healthcare provider and pharmacist about all of the medicines you take, including all prescription and non-prescription medicines such as over-the-counter medicines, supplements, or herbal remedies.
What are the possible side effects of CINRYZE?
Allergic reactions may occur with CINRYZE. Call your healthcare provider or get emergency support services right away if you have any of the following symptoms:
- wheezing
- difficulty breathing
- chest tightness
- turning blue (look at lips and gums)
- fast heartbeat
- swelling of the face
- faintness
- rash
- hives
In clinical studies, the most common side effects seen with CINRYZE were upper respiratory tract infections, sinusitis, rash, and headache.
These are not all the possible side effects of CINRYZE.
Tell your healthcare provider about any side effect that bothers you or that does not go away. You can also report side effects to the FDA at 1-800-FDA-1088.
You can ask your healthcare provider for information that is written for healthcare providers.
How should I store CINRYZE?
Do not freeze CINRYZE.
Store CINRYZE in a refrigerator or at room temperature between 36° to 77°F (2° to 25°C).
Keep CINRYZE in the original carton to protect it from light.
Do not use CINRYZE after the expiration date on the vial.
After preparing CINRYZE, you can store it at room temperature for up to 3 hours. If you have not used it within 3 hours, throw it away.
Only use the dissolved CINRYZE if it is colorless to slightly blue, clear and free from visible particles.
What else should I know about CINRYZE?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use CINRYZE for a condition for which it is not prescribed. Do not share CINRYZE with other people, even if they have the same symptoms that you have.
Because CINRYZE is made from human blood, it may carry a risk of transmitting infectious agents, e.g. viruses, and, theoretically, the Creutzfeldt-Jakob (CJD) agent.
This leaflet summarizes the most important information about CINRYZE. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about CINRYZE that was written for healthcare professionals.
Instructions for Use
Do not attempt to self-administer unless you have been taught how by your healthcare provider.
See the step-by-step instructions for injecting CINRYZE at the end of this leaflet. You should always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using CINRYZE. If you are unsure of the steps, please call your healthcare provider or pharmacist before using.
Call your healthcare provider right away if swelling is not controlled after using CINRYZE.
Your healthcare provider will prescribe the dose that you should take.
Call your healthcare provider if you take too much CINRYZE.
Call your healthcare provider if you miss a dose of CINRYZE.
Talk to your healthcare provider before traveling. You should plan to bring enough CINRYZE for your treatment during this time.
Preparation of CINRYZE
Always wash your hands before doing the following steps. Try to keep everything clean and germ-free while you are reconstituting CINRYZE. Once you open the vials, you should finish preparing CINRYZE as soon as possible. This will help to keep them germ-free.
Note: Two vials of CINRYZE are required for each dose. You should reconstitute both vials according to steps 1 through 10.
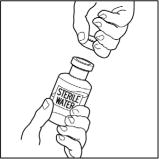
- Let the vial of CINRYZE and the vial of Sterile Water for Injection, USP reach room temperature.
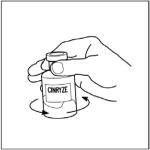
- Remove the cap from the CINRYZE vial and Sterile Water for Injection, USP vial to show the center part of the rubber stopper.
- Wipe the top of each vial with an alcohol wipe or swab, and allow it to dry. Do not blow on the stopper to dry it faster. Place each vial on a flat surface. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface.
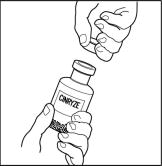
- Remove the protective covering from the shorter end of the double-ended transfer needle, place the vial on a flat surface, and insert the exposed needle through the center of the vial of Sterile Water for Injection, USP. While holding the needle in the vial, remove the protective covering from the longer end of the double-ended transfer needle.
- Turn the vial containing Sterile Water for Injection, USP, upside-down and quickly insert the free end of the needle through the center of the stopper of a CINRYZE vial. Hold the Sterile Water for Injection, USP vial at a slight angle, allowing the diluent to flow down the side of the CINRYZE vial to prevent foaming. The vacuum in the vial will draw the Sterile Water for Injection, USP, into the CINRYZE vial. If this does not happen, do not use the product.
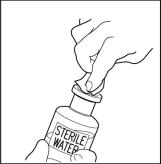
- Once all the Sterile Water for Injection, USP, is in the CINRYZE vial, disconnect the two vials by removing the needle from the CINRYZE vial stopper. Discard the vial that contained the Sterile Water for Injection, USP along with the transfer needle directly into a sharps container. Gently swirl the CINRYZE container until all the powder is dissolved. Do not shake.
Look at the final solution before using it to make sure that CINRYZE is completely dissolved. Once dissolved, the solution in the CINRYZE vial should be colorless to slightly blue and clear. Do not use the product if the solution is cloudy, discolored or contains any particles. Throw it away and prepare a new vial of CINRYZE.
One vial of dissolved CINRYZE contains 5 mL of C1 esterase inhibitor at a concentration of 100 Units/mL. Prepare two vials of CINRYZE for one dose.
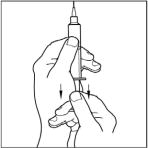
- Attach the filter needle to a sterile, disposable 10mL syringe and draw back the plunger to allow approximately 5mL of air into the syringe.
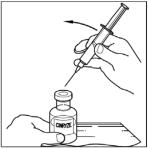
- Insert the filter needle into the vial of dissolved CINRYZE on a flat surface.
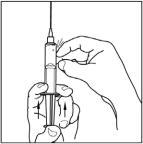
- Turn the vial of CINRYZE upside down, inject air into the vial (not into the solution), and then slowly pull as much CINRYZE solution as possible into the syringe. Withdraw the needle and syringe from the vial and remove any air bubbles by gently tapping the syringe with your finger and slowly pushing the air out of the syringe.
-
Remove and throw away the filter needle in a hard-walled sharps container for proper disposal.
Filter needles are meant to filter the contents of a single dose (2 vials) of CINRYZE only.
Repeat steps 1-10 above with a second vial of CINRYZE to make one complete dose of 10mL.
CINRYZE should be administered within 3 hours after preparation. The dissolved CINRYZE solution may be stored at room temperature prior to administration. If not used within 3 hours after preparation, throw away the CINRYZE solution.
SELF ADMINISTRATION (Intravenous Injection)
Your healthcare provider will teach you how to safely administer CINRYZE. It is important that CINRYZE is injected directly into a superficial vein and not injected into surrounding tissues and not injected into an artery. Once you learn how to self-administer, you can follow the instructions in this insert.
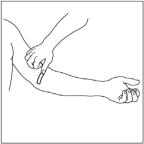
- Attach a needle or infusion set with a winged adapter to the syringe containing the dissolved CINRYZE solution.
- Apply a tourniquet and prepare the injection site by wiping the skin well with an alcohol swab.
- As instructed by your healthcare provider:
- Insert the butterfly needle of the infusion set tubing into your vein.
- Remove the tourniquet.
- Make sure that the needle is in a vein and that all the air has been removed from the tubing.
- In ject the dissolved CINRYZE product slowly over ten minutes (approximately 1mL/min).
- After infusing CINRYZE, remove the infusion set and discard. The amount of drug product left in the infusion set will not affect your treatment. Dispose of all unused solution, the empty vials, and the used needles and syringe in an appropriate container used for throwing away waste that might hurt others if not handled properly.
It is a good idea to record the lot number from the CINRYZE vial label every time you use CINRYZE.
This Patient Package Insert has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Sanquin Blood Supply Foundation
Amsterdam, The Netherlands
Distributed by:
ViroPharma Biologics, Inc.
Exton, PA 19341
U.S. License Number 1833
Vial Label
FOR INTRAVENOUS INJECTION
49-226
C1 esterase inhibitor (human)
ClNRYZETM
500 u
NDC 42227-081-05
Reconstitute with 5 mL Sterile Water for Injection, USP.
Use entire contents within 3 hours of reconstitution.
Single-use vial • Rx only • US License No. 1833
Manufactured by Sanquin Blood Supply Foundation, Amsterdam,
Netherlands 1006 AD, for ViroPharma Biologics, Inc., Exton, PA 19341
Carton Label
FOR INTRAVENOUS INJECTION
C1 esterase inhibitor (human)
ClNRYZETM
500 U
Contains 1 single-use vial.
Recommended dose is 1000 U
(2 vials).
Rx only
NDC 42227-081-05
See package insert for full prescribing
information and administration.
ViroPharma Biologics, Inc.
| CINRYZE
human c1 esterase inhibitor injection, powder, lyophilized, for solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA125267 | 12/01/2008 | |
| Labeler - ViroPharma Biologics, Inc. (832763085) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sanquin Blood Products Foundation, Plasma Products Division | 387373046 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| C.A.F.-D.C.F. cvba-scrl: Centrale Afdeling voor Fractionering van het Rode Kruis cvba - Departement Central de Fractionnement de la Croix-Rouge S.C.R.L | 375250156 | MANUFACTURE | |