AMPROLIUM P
-
amprolium solution
Teva Animal Health, Inc.
----------
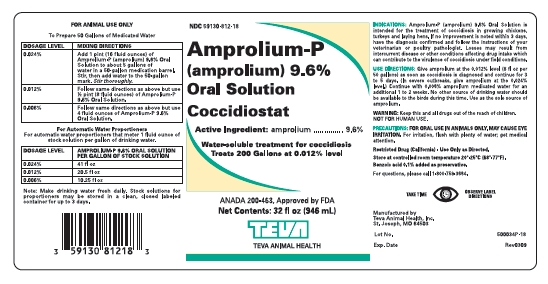
Amprolium-P(amprolium) 9.6%
Oral Solution
Coccidiostat
Active ingredient
amprolium …………… 9.6%
INDICATIONS
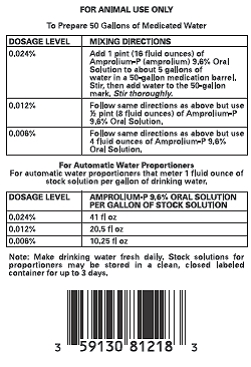
Amprolium-P (amprolium) 9.6% Oral Solution is intended for the treatment of coccidiosis in growing chickens, turkeys and laying hens. If no improvement is noted within 3 days, have the diagnosis confirmed and follow the instructions of your veterinarian or poultry pathologist. Losses may result from intercurrent disease or other conditions affecting drug intake which can contribute to the virulence of coccidiosis under field conditions.
USE DIRECTIONS:
Give amprolium at the 0.012% level (8 fl oz per 50 gallons) as soon as coccidiosis is diagnosed and continue for 3 to 5 days. (In severe outbreaks, give amprolium at the 0.024% level.) Cointinue with 0.006% amprolium medicated water for an addition 1 to 2 weeksl;. No other source of drinking water should be available to the birds during this time.l Use as sole source of amprolium see WARNING
WARNING
Keep this and all drugs out of the reach of children. NOT FOR HUMAN USE
PRECAUTIONS
FOR ORAL USE IN ANIMALS ONLY. MAY CAUSE EYE IRRITATION. For irritation, flush with plenty of water; get medical attention.
Restricted Drug (California)-Use only as Directed
Store at controlled room temperatures 20°-25°C (68°-77°F).
Benzoic acid 0.1% added as preservative
For questions, please call 1-800-759-3664

Manufactured by Teva Animal Health, Inc.
St. Joseph, MO. 64503
500034P
Rev0309
Lot No.
Exp. Date
Principle Display Panel
| AMPROLIUM P
amproliium p solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANADA | ANADA200463 | 11/11/2009 | |
| Labeler - Teva Animal Health, Inc. (625254461) |