GUERLAIN TERRACOTTA GLOSS 03 GRENADE- octinoxate lipstick
Guerlain S.A.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
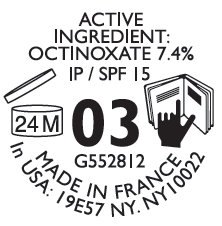
Active Ingredient
OCTINOXATE (ETHYLHEXYL METHOXYCINNAMATE) 7.4 %
Other Ingredients
TRIMETHYLOLPROPANE TRIISOSTEARATE, METHYL HYDROGENATED ROSINATE, DIMER DILINOLEYL DIMER DILINOLEATE, DIISOSTEARYL MALATE, TRIDECYL TRIMELLITATE, C18-36 ACID TRIGLYCERIDE, SYNTHETIC FLUORPHLOGOPITE, SILICA DIMETHYL SILYLATE, SCLEROCARYA BIRREA SEED OIL, PRUNUS ARMENIACA (APRICOT) KERNEL OIL, PARFUM (FRAGRANCE), PROPYL GALLATE, BENZYL BENZOATE, BHT, TOCOPHEROL, ALUMINUM HYDROXIDE, ALPHA-ISOMETHYL IONONE, LIMONENE, SOLANUM LYCOPERSICUM (TOMATO) FRUIT/LEAF/STEM EXTRACT, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT[ C177891 (TITANIUM DIOXIDE), C1 77491, C1 77492, C1 77499 (IRON OXIDES), C1 15850 (RED 6, RED 6 LAKE, RED 7, RED 7 LAKE), C1 10140 (YELLOW 5 LAKE), C1 15985 (YELLOW 6 LAKE), C1 45410 (RED 28 LAKE), C1 42090 (BLUE 1 LAKE), C1 73360 (RED 30 LAKE), C1 45380 (RED 21 LAKE), C1 17200 (RED 33 LAKE), C1 77742 (MANGANESE VIOLET), C1 77163 (BISMUTH OXYCHLORIDE)]
Warning
FOR EXTERNAL USE ONLY. AVOID CONTACT WITH EYES.
Discontinue Use If Signs Of Irritation Appear
KEEP OUT OF REACH OF CHILDREN
Guerlain S.A.