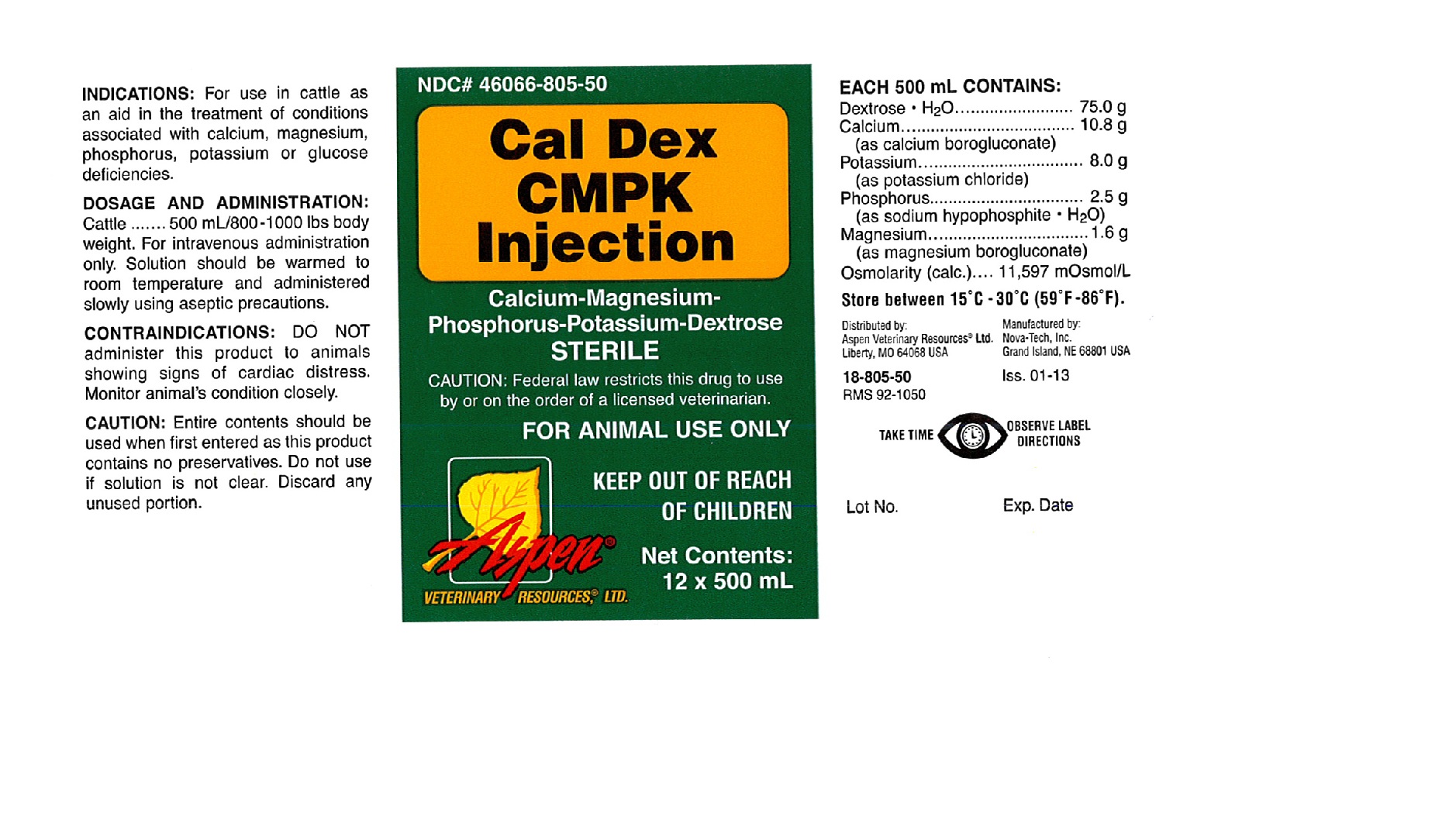
CAL DEX CMPK- dextrose monohydrate, potassium chloride, calcium gluconate monohydrate, phosphorus and magnesium gluconate injection, solution
Aspen Veterinary
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Cal Dex CMPK Injection
INDICATIONS:
For use in cattle as an aid in the treatment of conditions associated with calcium, magnesium, phosphorus, potassium or glucose deficiencies.
DOSAGE AND ADMINISTRATION:
Cattle ....... 500 mL/800-1000 lbs body weight. For intravenous administration only. Solution should be warmed to room temperature and administered slowly using aseptic precautions.
CONTRAINDICATIONS:
DO NOT administer this product to animals showing signs of cardiac distress. Monitor animal's condition closely.
CAUTION:
Entire contents should be used when first entered as this product contains no preservatives. Do not use if solution is not clear. Discard any unused portion.
EACH 500 mL CONTAINS:
Dextrose.H2O ........................ 75.0 g
Calcium ................................ 10.8 g
(as calcium borogluconate)
Potassium ............................. 8 .0 g
(as potassium chloride)
Phosphorus ........................... 2.5 g
(as sodium hypophosphite.H2O)
Magnesium ............................ 1.6 g
(as magnesium borogluconate)
Oslolarity (calc.).... 11,597 mOsmol/L
| CAL DEX CMPK
cal dex cmpk injection, solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Aspen Veterinary (627265361) |
| Registrant - Aspen Veterinary (627265361) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nova-Tech, Inc. | 196078976 | api manufacture | |