methadone hydrochloride (Methadone Hydrochloride) tablet
[Mallinckrodt Inc.]
CII
Rx only.
CONDITIONS FOR DISTRIBUTION AND USE OF METHADONE PRODUCTS:
Code of Federal Regulations,
Title 21, Sec. 291.505
METHADONE PRODUCTS, WHEN USED FOR TREATMENT OF NARCOTIC ADDICTION IN DETOXIFICATION OR MAINTENANCE PROGRAMS, SHALL BE DISPENSED ONLY BY APPROVED HOSPITAL PHARMACIES, APPROVED COMMUNITY PHARMACIES, AND MAINTENANCE PROGRAMS APPROVED BY THE FOOD AND DRUG ADMINISTRATION AND THE DESIGNATED STATE AUTHORITY. APPROVED MAINTENANCE PROGRAMS SHALL DISPENSE AND USE METHADONE IN ORAL FORM ONLY AND ACCORDING TO THE TREATMENT REQUIREMENTS STIPULATED IN THE FEDERAL METHADONE REGULATIONS (21 CFR 291.505). FAILURE TO ABIDE BY THE REQUIREMENTS IN THESE REGULATIONS MAY RESULT IN CRIMINAL PROSECUTION, SEIZURE OF THE DRUG SUPPLY, REVOCATION OF THE PROGRAM APPROVAL, AND INJUNCTION PRECLUDING OPERATION OF THE PROGRAM. A METHADONE PRODUCT, WHEN USED AS AN ANALGESIC, MAY BE DISPENSED IN ANY LICENSED PHARMACY.
DESCRIPTION
Methadone Hydrochloride Tablets, USP are provided in tablet form for oral administration. Methadone Hydrochloride, USP 6-(dimethylamino)-4, 4-diphenyl-3-heptanone hydrochloride, is a white, essentially odorless, bitter-tasting powder. It is very soluble in water, soluble in isopropanol and in chloroform, and practically insoluble in ether and in glycerine. Methadone hydrochloride has a pKa of 8.25 in water at 20°C. Its molecular weight is 345.91 and it has the following structural formula.
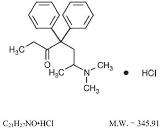
Each tablet contains 5 mg (0.015 mmol) or 10 mg (0.029 mmol) methadone hydrochloride.
The tablets also contain Lactose Monohydrate NF, Magnesium Stearate NF, Microcrystalline Cellulose NF and Silicon Dioxide NF.
CLINICAL PHARMACOLOGY
Methadone hydrochloride is a synthetic narcotic analgesic with multiple actions quantitatively similar to those of morphine, the most prominent of which involve the central nervous system and organs composed of smooth muscle. The principal actions of therapeutic value are analgesia and sedation and detoxification or temporary maintenance in narcotic addiction. The methadone abstinence syndrome, although qualitatively similar to that of morphine, differs in that the onset is slower, the course is more prolonged, and the symptoms are less severe.
A parenteral dose of 8 to 10 mg of methadone is approximately equivalent in analgesic effect to 10 mg of morphine. With single-dose administration, the onset and duration of analgesic action of the 2 drugs are similar.
When administered orally, methadone is approximately one-half as potent as when given parenterally. Oral administration results in a delay of the onset, a lowering of the peak, and an increase in the duration of analgesic effect.
INDICATIONS AND USAGE
(see boxed note)
For relief of severe pain.
For detoxification treatment of narcotic addiction.
For temporary maintenance treatment of narcotic addiction.
NOTE
If methadone is administered for treatment of heroin dependence for more than 3 weeks, the procedure passes from treatment of the acute withdrawal syndrome (detoxification) to maintenance therapy. Maintenance treatment is permitted to be undertaken only by approved methadone programs. This does not preclude the maintenance treatment of an addict who is hospitalized for medical conditions other than addiction and who requires temporary maintenance during the critical period of his/her stay or whose enrollment has been verified in a program approved for maintenance treatment with methadone.
CONTRAINDICATIONS
Hypersensitivity to methadone.
WARNINGS
Methadone Hydrochloride Tablets, USP are for oral administration only and must not be used for injection. It is required that Methadone Hydrochloride Tablets, USP, if dispensed, be packaged in child-resistant containers and kept out of the reach of children to prevent accidental ingestion.
Interaction With Other Central Nervous System Depressants— Methadone should be used with caution and in reduced dosage in patients who are concurrently receiving other narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics, tricyclic antidepressants, and other CNS depressants (including alcohol). Respiratory depression, hypotension, and profound sedation or coma may result.
Anxiety— Since methadone, as used by tolerant subjects at a constant maintenance dosage, is not a tranquilizer, patients who are maintained on this drug will react to life problems and stresses with the same symptoms of anxiety as do other individuals. The physician should not confuse such symptoms with those of narcotic abstinence and should not attempt to treat anxiety by increasing the dosage of methadone. The action of methadone in maintenance treatment is limited to the control of narcotic symptoms and is ineffective for relief of general anxiety.
Head Injury and Increased Intracranial Pressure— The respiratory depressant effects of the methadone and its capacity to elevate cerebrospinal-fluid pressure may be markedly exaggerated in the presence of increased intracranial pressure. Furthermore, narcotics produce side effects that may obscure the clinical course of patients with head injuries. In such patients, methadone must be used with caution and only if it is deemed essential.
Asthma and Other Respiratory Conditions— Methadone should be used with caution in patients having an acute asthmatic attack, in those with chronic obstructive pulmonary disease or cor pulmonale, and in individuals with a substantially decreased respiratory reserve, preexisting respiratory depression, hypoxia, or hypercapnia. In such patients, even usual therapeutic doses of narcotics may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea.
Hypotensive Effect— The administration of methadone may result in severe hypotension in an individual whose ability to maintain normal blood pressure has already been compromised by a depleted blood volume or concurrent administration of such drugs as the phenothiazines or certain anesthetics.
Use in Ambulatory Patients— Methadone may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
Methadone, like other narcotics, may produce orthostatic hypotension in ambulatory patients.
Use in Pregnancy— Safe use in pregnancy has not been established in relation to possible adverse effects on fetal development. Therefore, methadone should not be used in pregnant women unless, in the judgment of the physician, the potential benefits outweigh the possible hazards.
Methadone is not recommended for obstetric analgesia because its long duration of action increases the probability of respiratory depression in the newborn.
Use in Children— Methadone is not recommended for use as an analgesic in children, since documented clinical experience has been insufficient to establish a suitable dosage regimen for the pediatric age group.
PRECAUTIONS
Drug Interactions:
Pentazocine—Patients who are addicted to heroin or who are on the methadone maintenance program may experience withdrawal symptoms when given an opioid agonist-antagonist, such as pentazocine.
Rifampin— The concurrent administration of rifampin may possibly reduce the blood concentration of methadone to a degree sufficient to produce withdrawal symptoms. The mechanism by which rifampin may decrease blood concentrations of methadone is not fully understood although enhanced microsomal drug-metabolized enzymes may influence drug disposition.
Monoamine Oxidase (MAO) Inhibitors— Therapeutic doses of meperidine have precipitated severe reactions in patients concurrently receiving monoamine oxidase inhibitors or those who have received such agents within 14 days. Similar reactions thus far have not been reported with methadone; but if the use of methadone is necessary in such patients, a sensitivity test should be performed in which repeated small incremental doses are administered over the course of several hours while the patient's condition and vital signs are under careful observation.
Desipramine— Blood levels of desipramine have increased with concurrent methadone therapy.
Special-Risk Patients— Methadone should be given with caution and the initial dose should be reduced in certain patients, such as the elderly or debilitated and those with severe impairment of hepatic or renal function, hypothyroidism, Addison's disease, prostatic hypertrophy, or urethral stricture.
Acute Abdominal Conditions— The administration of methadone or other narcotics may obscure the diagnosis or clinical course in patients with acute abdominal conditions.
ADVERSE REACTIONS
THE MAJOR HAZARDS OF METHADONE, AS OF OTHER NARCOTIC ANALGESICS, ARE RESPIRATORY DEPRESSION AND, TO A LESSER DEGREE, CIRCULATORY DEPRESSION. RESPIRATORY ARREST, SHOCK, AND CARDIAC ARREST HAVE OCCURRED.
The most frequently observed adverse reactions include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not suffering severe pain. In such individuals, lower doses are advisable. Some adverse reactions may be alleviated if the ambulatory patient lies down.
Other adverse reactions include the following:
Central Nervous System— Euphoria, dysphoria, weakness, headache, insomnia, agitation, disorientation, and visual disturbances.
Gastrointestinal— Dry mouth, anorexia, constipation and biliary tract spasm.
Cardiovascular— Flushing of the face, bradycardia, palpitation, faintness, and syncope.
Genitourinary— Urinary retention or hesitancy, antidiuretic effect, and reduced libido and/or potency.
Allergic— Pruritus, urticaria, other skin rashes, edema, and, rarely, hemorrhagic urticaria.
Hematologic— Reversible thrombocytopenia has been described in a narcotics addict with chronic hepatitis.
DRUG ABUSE AND DEPENDENCE
Methadone hydrochloride, a narcotic, is a Schedule II controlled substance under the Federal Controlled Substances Act. Appropriate security measures should be taken to safeguard stocks of methadone against diversion.
DRUG DEPENDENCE— METHADONE CAN PRODUCE DRUG DEPENDENCE OF THE MORPHINE TYPE AND, THEREFORE, HAS THE POTENTIAL FOR BEING ABUSED. PSYCHIC DEPENDENCE, PHYSICAL DEPENDENCE, AND TOLERANCE MAY DEVELOP ON REPEATED ADMINISTRATION OF METHADONE, AND IT SHOULD BE PRESCRIBED AND ADMINISTERED WITH THE SAME DEGREE OF CAUTION APPROPRIATE TO THE USE OF MORPHINE.
OVERDOSAGE
Signs and Symptoms— Methadone is an opioid and produces effects similar to those of morphine. Symptoms of overdose begin within seconds after intravenous administration and within minutes of nasal, oral, or rectal administration. Prominent symptoms are miosis, respiratory depression, somnolence, coma, cool clammy skin, skeletal muscle flaccidity that may progress to hypotension, apnea, bradycardia, and death. Noncardiac pulmonary edema may occur and monitoring of heart filling pressures may be helpful.
Treatment— To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
Initial management of opioid overdose should include establishment of a secure airway and support of ventilation and perfusion. Naloxone may be given to antagonize opioid effects, but the airway must be secured as vomiting may ensue. The duration of methadone effect is much longer (36 to 48 hours) than the duration of naloxone effect (1 to 3 hours) and repeated doses (or continuous intravenous infusion) of naloxone may be required.
If the patient has chronically abused opioids, administration of naloxone may precipitate a withdrawal syndrome that may include yawning, tearing, restlessness, sweating, dilated pupils, piloerection, vomiting, diarrhea, and abdominal cramps. If these symptoms develop, they should abate quickly as the effects of naloxone dissipate.
If methadone has been taken by mouth, protect the patient's airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient's vital signs, blood gases, serum electrolytes, etc. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient's airway when employing gastric emptying or charcoal.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of methadone.
NOTE
IN AN INDIVIDUAL PHYSICALLY DEPENDENT ON NARCOTICS, THE ADMINISTRATION OF THE USUAL DOSE OF A NARCOTIC ANTAGONIST WILL PRECIPITATE AN ACUTE WITHDRAWAL SYNDROME. THE SEVERITY OF THIS SYNDROME WILL DEPEND ON THE DEGREE OF PHYSICAL DEPENDENCE AND THE DOSE OF THE ANTAGONIST ADMINISTERED. THE USE OF A NARCOTIC ANTAGONIST IN SUCH A PERSON SHOULD BE AVOIDED IF POSSIBLE. IF IT MUST BE USED TO TREAT SERIOUS RESPIRATORY DEPRESSION IN THE PHYSICALLY DEPENDENT PATIENT, THE ANTAGONIST SHOULD BE ADMINISTERED WITH EXTREME CARE AND BY TITRATION WITH SMALLER THAN USUAL DOSES OF THE ANTAGONIST.
DOSAGE AND ADMINISTRATION
For Relief of Pain— Dosage should be adjusted according to the severity of the pain and the response of the patient. Occasionally, it may be necessary to exceed the usual dosage recommended in cases of exceptionally severe pain or in those patients who have become tolerant to the analgesic effects of narcotics.
The usual adult dosage is 2.5 mg to 10 mg every three or four hours as necessary.
For Detoxification Treatment— THE DRUG SHALL BE ADMINISTERED DAILY UNDER CLOSE SUPERVISION AS FOLLOWS:
A detoxification treatment course shall not exceed 21 days and may not be repeated earlier than 4 weeks after completion of the preceding course.
In detoxification, the patient may receive methadone when there are significant symptoms of withdrawal. The dosage schedules indicated below are recommended but could be varied in accordance with clinical judgment. Initially, a single oral dose of 15 to 20 mg of methadone will often be sufficient to suppress withdrawal symptoms. Additional methadone may be provided if withdrawal symptoms are not suppressed or if symptoms reappear. When patients are physically dependent on high doses, it may be necessary to exceed these levels. Forty mg/day in single or divided doses will usually constitute an adequate stabilizing dosage level. Stabilization can be continued for 2 to 3 days, and then the amount of methadone normally will be gradually decreased. The rate at which methadone is decreased will be determined separately for each patient. The dose of methadone can be decreased on a daily basis or at 2-day intervals, but the amount of intake shall always be sufficient to keep withdrawal symptoms at a tolerable level. In hospitalized patients, a daily reduction of 20% of the total daily dose may be tolerated and may cause little discomfort. In ambulatory patients, a somewhat slower schedule may be needed. If methadone is administered for more than 3 weeks, the procedure is considered to have progressed from detoxification or treatment of the acute withdrawal syndrome to maintenance treatment, even though the goal and intent may be eventual total withdrawal.
If the patient is unable to ingest oral medication, parenteral administration may be substituted.
HOW SUPPLIED
Each Methadone Hydrochloride, USP 5 mg tablet contains 5 mg Methadone Hydrochloride, USP. It is available as a white to off-white, modified rectangle shaped convex tablet, one side debossed with a score between “57” and “55”;  on the other side.
on the other side.
Bottles of 100 ................NDC No. 0406-5755-01
Unit Dose (10x10)..........NDC No. 0406-5755-62
Each Methadone Hydrochloride, USP 10 mg tablet contains 10 mg Methadone Hydrochloride, USP. It is available as a white to off-white, modified rectangle shaped convex tablet, one side debossed with a score between “57” and “71”;  on the other side.
on the other side.
Bottles of 100 ...............NDC No. 0406-5771-01
Unit Dose (10x10).........NDC No. 0406-5771-62
Dispense in a tight, light-resistant container as defined in the USP/NF.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
 is a registered trademark of Mallinckrodt Inc.
is a registered trademark of Mallinckrodt Inc.
tyco
Healthcare
Mallinckrodt
Mallinckrodt Inc.
St. Louis, Missouri 63134, U.S.A. Rev. 092304
| Methadone Hydrochloride (Methadone Hydrochloride) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Methadone Hydrochloride (Methadone Hydrochloride) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 06/2006