condylox (podofilox) gel
[Oclassen Dermatologics]
DESCRIPTION
Podofilox is an antimitotic drug which can be chemically synthesized or purified from the plant families Coniferae and Berberidaceae (e.g. species of Juniperus and Podophyllum). Condylox® Gel 0.5% is formulated for topical administration. Each gram of gel contains 5 mg of podofilox in a buffered alcoholic gel containing alcohol, glycerin, lactic acid, hydroxypropyl cellulose, sodium lactate, and butylated hydroxytoluene.
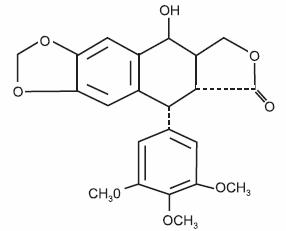
Podofilox has a molecular weight of 414.4 daltons, and is soluble in alcohol and sparingly soluble in water. Its chemical name is [5R,-(5α, 5aβ, 8aα, 9α]-5,8,8a,9-tetrahydro-9-hydroxy-5-(3,4,5-trimethoxyphenyl)furo[3',4':6,7]naphtho-[2,3,-d]-1,3-dioxol-6(5aH)-one.
Podofilox has the following structural formula:
CLINICAL PHARMACOLOGY
Mechanism of Action
Treatment of anogenital warts with podofilox results in necrosis of visible wart tissue. The exact mechanism of action is unknown.
Pharmacokinetics
In systemic absorption studies in 52 patients, topical application of 0.05 mL of an ethanolic solution containing 0.5% podofilox to external genitalia did not result in detectable serum levels. Applications of 0.1 to 1.5 mL resulted in peak serum levels of 1 to 17 ng/mL one to two hours after application. The elimination half-life ranged from 1.0 to 4.5 hours. The drug was not found to accumulate after multiple treatments1.
CLINICAL STUDIES
In the first multicenter clinical study in 326 patients with anogenital warts, Condylox® Gel 0.5% and its vehicle were applied in a double-blind fashion to comparable patient groups. Of the 260 patients with efficacy data, 176 were treated with Condylox® Gel 0.5%. Patients applied Condylox® Gel 0.5% twice daily for three consecutive days followed by a 4 day “rest” period.
At the end of 4 weeks, 38.4% of the patients had complete clearing of the wart tissue when treated with Condylox® Gel 0.5%.
In the second multicenter clinical trial in 108 evaluable patients with anogenital warts, Condylox® (podofilox) Topical Solution 0.5% was compared with Condylox® Gel 0.5% for efficacy. As in the first clinical trial, patients applied Condylox® Gel 0.5% twice daily for three consecutive days followed by a four day “rest” period.
Similar clearance rates were observed. At the end of 4 weeks, 25.6% of the patients had complete clearing of the wart tissue when treated with Condylox® Gel 0.5%
INDICATIONS AND USAGE
Condylox® Gel 0.5% is indicated for the topical treatment of anogenital warts (external genital warts and perianal warts). This product is not indicated in the treatment of mucous membrane warts (see PRECAUTIONS).
Diagnosis
Although anogenital warts have a characteristic appearance, histopathologic confirmation should be obtained if there is any doubt of the diagnosis. Differentiating warts from squamous cell carcinoma and "Bowenoid papulosis" is of particular concern. Squamous cell carcinoma may also be associated with human papillomavirus which should not be treated with Condylox® Gel 0.5%.
CONTRAINDICATIONS
Condylox® Gel 0.5% is contraindicated for patients who develop hypersensitivity or intolerance to any components of the formulation.
WARNINGS
Correct diagnosis of the lesions to be treated is essential. See the Diagnosis subsection of the INDICATIONS AND USAGE section. Condylox® Gel 0.5% is intended for cutaneous use only. Avoid contact with the eyes. If contact with the eyes occurs, patients should immediately flush the eyes with copious quantities of water and seek medical advice.
Drug Product is Flammable.
Keep Away From Open Flame.
PRECAUTIONS
General
Data are not available on the safe and effective use of this product for treatment of warts occurring on mucous membranes of the genital area (including the urethra, rectum and vagina). The recommended method of application, frequency of application, and duration of usage should not be exceeded (see DOSAGE AND ADMINISTRATION).
Information for Patients
Patients using Condylox® Gel 0.5% should receive the following information and instructions. This information is intended to aid in the safe and effective use of this medication. It is not intended to disclose all possible adverse or intended effects.
-
This medication should be used only as directed by the health care provider. Patients should be instructed to wash their hands thoroughly before and after each application. It is for external use only. Avoid contact with the eyes.
-
Patients should be advised not to use this medication for any disorder other than for which it was prescribed.
-
Patients should report any signs of adverse reactions to the health care provider.
-
If no improvement is observed after 4 weeks of treatment, discontinue the medication and consult the health care provider.
Carcinogenesis, Mutagenesis and Impairment of Fertility
An 80-week carcinogenicity study in the mouse was performed using a 0.5% podofilox solution applied dermally at 0.04, 0.2 and 1.0 mg/kg/day. There were no differences between the podofilox treated mice at any dose level and vehicle control in the incidence of neoplasia. Published animal studies, in general, have not shown the drug substance, podofilox, to be carcinogenic.2,3,4,5,6 There are published reports that, in mouse studies, crude podophyllin resin (containing podofilox) applied topically to the cervix produced changes resembling carcinoma in situ.7 These changes were reversible at five weeks after cessation of treatment. In one reported experiment, epidermal carcinoma of the vagina and cervix was found in 1 out of 18 mice after 120 applications of podophyllin8 (the drug was applied twice weekly over a 15-month period).
Podofilox was not mutagenic in the Ames plate reverse mutation assay at concentrations up to 5 mg/plate, with and without metabolic activation.
No cell transformation related to potential oncogenicity was observed in BALB/3T3 cells after exposure to podofilox at concentrations up to 0.008 μg/mL, without metabolic activation and 12 μg/mL podofilox with metabolic activation. Results from the mouse micronucleus in vivo assay using podofilox 0.5% solution at doses up to 25 mg/kg (75 mg/m2), indicate that podofilox should be considered a potential clastogen (a chemical that induces disruption and breakage of chromosomes).
Daily topical application of 0.5% podofilox solution at doses up to the equivalent of 0.2 mg/kg (1.18 mg/m2, approximately equivalent to the human daily dose) to rats throughout gametogenesis, mating, gestation, parturition and lactation for two generations demonstrated no impairment of fertility.
Pregnancy
Pregnancy Category C: 0.5% podofilox solution was not teratogenic in the rabbit following topical application of up to 0.21 mg/kg (2.85 mg/m2, approximately 2 times the maximum human dose) once daily for 13 days. The scientific literature contains references that podofilox is embryotoxic in rats when administered intraperitoneally at a dose of 5 mg/kg (29.5 mg/m2, approximately 19 times the recommended maximum human dose.)9 Teratogenicity and embryotoxicity have not been studied with intravaginal application. Many antimitotic drug products are known to be embryotoxic. There are no adequate and well-controlled studies in pregnant women. Condylox® Gel 0.5% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from podofilox, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS
In clinical trials with Condylox® Gel 0.5%, the following local adverse reactions were reported during the treatment of anogenital warts. The severity of local adverse reactions were predominantly mild or moderate and did not increase during the treatment period. Severe reactions were most frequent within the first 2 weeks of treatment.
| Adverse Reaction | Mild | Moderate | Severe |
| Inflammation | 32.2% | 30.4% | 9.3% |
| Burning | 37.1% | 25.9% | 11.5% |
| Erosion | 27.0% | 20.8% | 8.9% |
| Pain | 23.7% | 20.4% | 11.5% |
| Itching | 32.2% | 16.0% | 7.8% |
| Bleeding | 19.2% | 3.0% | 0.7% |
Other local adverse reactions reported included stinging (7%), and erythema (5%); less commonly reported local adverse events included desquamation, scabbing, discoloration, tenderness, dryness, crusting, fissures, soreness, ulceration, swelling/edema, tingling, rash, and blisters.
The most common systemic adverse event reported during the clinical studies was headache (7%).
OVERDOSAGE
Topically applied podofilox may be absorbed systemically (see CLINICAL PHARMACOLOGY section). Toxicity reported following systemic administration of podofilox in investigational use for cancer treatment included: nausea, vomiting, fever, diarrhea, bone marrow depression, and oral ulcers. Following 5 to 10 daily intravenous doses of 0.5 to 1 mg/kg/day, significant hematological toxicity occurred but was reversible.10 Other toxicities occurred at lower doses. Toxicity reported following systemic administration of podophyllum resin included: nausea, vomiting, fever, diarrhea, peripheral neuropathy, altered mental status, lethargy, coma, tachypnea, respiratory failure, leukocytosis, pancytosis, hematuria, renal failure and seizures.11 Treatment of topical overdosage should include washing the skin free of any remaining drug and symptomatic and supportive therapy.
DOSAGE AND ADMINISTRATION
The prescriber should ensure that the patient is fully aware of the correct method of therapy and identify which specific warts should be treated.
Apply twice daily for 3 consecutive days, then discontinue for 4 consecutive days. This one week cycle of treatment may be repeated until there is no visible wart tissue or for a maximum of four cycles. If there is incomplete response after four treatment cycles, discontinue treatment and consider alternative treatment. Safety and effectiveness of more than four treatment cycles has not been established. There is no evidence to suggest that more frequent application will increase efficacy, but additional applications would be expected to increase the rate of local adverse reactions and systemic absorption.
Condylox® Gel 0.5% should be applied to the warts with the applicator tip or finger. Application on the surrounding normal tissue should be minimized. Treatment should be limited to 10 cm2 or less of wart tissue and to no more than 0.5 g of the gel per day.
Care should be taken to allow the gel to dry before allowing the return of opposing skin surfaces to their normal positions. Patients should be instructed to wash their hands thoroughly before and after each application.
HOW SUPPLIED
Condylox® Gel 0.5% is supplied as 3.5 g of clear gel in aluminum tubes with an applicator tip. NDC 55515-102-01. Store at controlled room temperature between 15 - 30°C (59 - 86°F). Avoid excessive heat. Do not freeze.
Rx only
REFERENCES
-
von Krogh G. Podophyllotoxin in serum: Absorption subsequent to three day repeated applications of a 0.5% ethanolic preparation on condylomata acuminata. Sex Trans Disease 1982: 9: 26-33.
-
Berenblum I. The effect of podophyllotoxin on the skin of the mouse, with reference to carcinogenic, cocarcinogenic, and anticarcinogenic action. J Cancer Inst 11:839-841, 1951.
-
Kaminetzky HA, Swerdlow M. Podophyllin and the mouse cervix: assessment of carcinogenic potential. Am J Obst Gyn 95:486-490, 1965.
-
McGrew EA, Kaminetzky HA. The genesis of experimental cervical epithelial dysplasia. Am J Clin Path 35:538-545, 1961.
-
Roe FJC, Salaman MH. Further studies on incomplete carcinogenesis: triethylene melamine (T.E.M.) 1,2 benxanthracene and betapropiolactone as initiators of skin tumor formation in the mouse. Brit J Cancer, 9:177-203, 1955.
-
Taper HS. Induction of the deficient acid DNAase activity in mouse interfollicular epidermis by croton oil as a possible tumor promoting mechanism. Zeitschrift fur Krebsforschung and Klinisch Onkologie (Cancer Research and Clinical Oncology, Berlin) 90:197-210, 1977.
-
Kaminetzky HA, McGrew EA, Phillips RL. Experimental cervical epithelial dysplasia. J Obst Gyn 14:1-10, 1959.
-
Kaminetzky HA, McGrew EA: Podophyllin and mouse cervix: Effect of long term application. Arch Path 73:481-485, 1962.
-
Thiersch JB. Effect of podophyllin (P) and podophylotoxine (PT.) on the rat litter in utero. Soc Exptl Biol Med Proc. 113:124-127, 1963.
-
Savel H.: Clinical experience with intravenous podophyllotoxin. Proc Amer Assoc Cancer Res, 1964; 5: 56.
-
Cassidy DE, Dewry J and Fanning JP: Podophyllum toxicity: A report of a fatal case and a review of the literature. J Toxicol Clinic Toxicol 1982: 19: 35-44.

Revised: OCTOBER 2005
128291-1005
| Condylox (podofilox) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
Revised: 05/2006Oclassen Dermatologics