dextroamphetamine sulfate (Dextroamphetamine Sulfate) capsule
[BARR LABORATORIES, INC.]

1009540103
Rx only
WARNING:
| AMPHETAMINES HAVE A HIGH POTENTIAL FOR ABUSE. ADMINISTRATION OF AMPHETAMINES FOR PROLONGED PERIODS OF TIME MAY LEAD TO DRUG DEPENDENCE AND MUST BE AVOIDED. PARTICULAR ATTENTION SHOULD BE PAID TO THE POSSIBILITY OF SUBJECTS OBTAINING AMPHETAMINES FOR NON-THERAPEUTIC USE OR DISTRIBUTION TO OTHERS, AND THE DRUGS SHOULD BE PRESCRIBED OR DISPENSED SPARINGLY. |
Description:
Dextroamphetamine sulfate is the dextro isomer of the compound d,l -amphetamine sulfate, a sympathomimetic amine of the amphetamine group. Chemically, dextroamphetamine is d-alpha-methylphenethylamine, and is present in all forms of dextroamphetamine as the neutral sulfate. The structural formula is as follows:
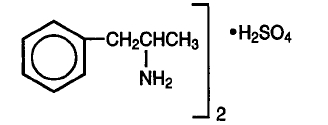
(C9H13N)2• H2SO4 Molecular Weight: 368.49
Each extended-release capsule is so prepared that an initial dose is released promptly and the remaining medication is released gradually over a prolonged period.
Each capsule contains dextroamphetamine sulfate, and has the following inactive ingredients: colloidal silicon dioxide, dibutyl sebacate, ethylcellulose aqueous dispersion, methylcellulose, povidone, propylene glycol, sugar spheres and talc.
The capsule shell ingredients in the 5 mg are D&C red no. 33, FD&C blue no. 1, FD&C yellow no. 6, gelatin, and titanium dioxide.
The capsule shell ingredients in the 10 mg are black iron oxide, gelatin, red iron oxide, titanium dioxide, and yellow iron oxide.
The capsule shell ingredients in the 15 mg are black iron oxide, gelatin, red iron oxide, titanium dioxide, and yellow iron oxide.
The imprinting ingredients are D&C yellow no. 10 aluminum lake, FD&C blue no. 1 aluminum lake, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, pharmaceutical glaze, propylene glycol, and synthetic black iron oxide.
Clinical PharmacOLOGY:
Amphetamines are non-catecholamine, sympathomimetic amines with CNS stimulant activity. Peripheral actions include elevations of systolic and diastolic blood pressures and weak bronchodilator and respiratory stimulant action.
There is neither specific evidence which clearly establishes the mechanism whereby amphetamines produce mental and behavioral effects in children, nor conclusive evidence regarding how these effects relate to the condition of the central nervous system.
Dextroamphetamine sulfate capsules are formulated to release the active drug substance in vivo in a more gradual fashion than the standard formulation, as demonstrated by blood levels. The formulation has not been shown superior in effectiveness over the same dosage of the standard, non-controlled-release formulations given in divided doses.
Pharmacokinetics:
The pharmacokinetics of the tablet and extended-release capsule was compared in 12 healthy subjects. The extent of bioavailability of the extended-release capsule was similar compared to the immediate release tablet. Following administration of three 5 mg tablets, average maximal dextroamphetamine plasma concentrations (Cmax) of 36.6 ng/mL were achieved at approximately 3 hours. Following administration of one 15 mg extended-release capsule, maximal dextroamphetamine plasma concentrations were obtained approximately 8 hours after dosing. The average Cmax was 23.5 ng/mL. The average plasma T1/2 was similar for both the tablet and extended-release capsule and was approximately 12 hours.
In 12 healthy subjects, the rate and extent of dextroamphetamine absorption were similar following administration of the extended-release capsule formulation in the fed (58 to 75 gm fat) and fasted state.
Indications and UsaGE:
Dextroamphetamine sulfate is indicated:
1. In Narcolepsy.
2. In Attention Deficit Disorder with Hyperactivity, as an integral part of a total treatment program which typically includes other remedial measures (psychological, educational, social) for a stabilizing effect in pediatric patients (ages 3 years to 16 years) with a behavioral syndrome characterized by the following group of developmentally inappropriate symptoms: moderate to severe distractibility, short attention span, hyperactivity, emotional lability, and impulsivity. The diagnosis of this syndrome should not be made with finality when these symptoms are only of comparatively recent origin. Nonlocalizing (soft) neurological signs, learning disability, and abnormal EEG may or may not be present, and a diagnosis of central nervous system dysfunction may or may not be warranted.
ContraindicaTIONS:
Advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension, hyperthyroidism, known hypersensitivity or idiosyncrasy to the sympathomimetic amines, glaucoma.
Agitated states.
Patients with a history of drug abuse.
During or within 14 days following the administration of monoamine oxidase inhibitors (hypertensive crises may result).
PrecautionS:
General:
Caution is to be exercised in prescribing amphetamines for patients with even mild hypertension.
The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
Information for Patients:
Amphetamines may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or vehicles; the patient should therefore be cautioned accordingly.
Drug Interactions:
Acidifying agents:
Gastrointestinal acidifying agents (guanethidine, reserpine, glutamic acid HCl, ascorbic acid, fruit juices, etc.) lower absorption of amphetamines. Urinary acidifying agents (ammonium chloride, sodium acid phosphate, etc.) increase the concentration of the ionized species of the amphetamine molecule, thereby increasing urinary excretion. Both groups of agents lower blood levels and efficacy of amphetamines.
Adrenergic blockers:
Adrenergic blockers are inhibited by amphetamines.
Alkalinizing agents:
Gastrointestinal alkalinizing agents (sodium bicarbonate, etc.) increase absorption of amphetamines. Urinary alkalinizing agents (acetazolamide, some thiazides) increase the concentration of the non-ionized species of the amphetamine molecule, thereby decreasing urinary excretion. Both groups of agents increase blood levels and therefore potentiate the actions of amphetamines.
Antidepressants, tricyclic:
Amphetamines may enhance the activity of tricyclic or sympathomimetic agents; d-amphetamine with desipramine or protriptyline and possibly other tricyclics cause striking and sustained increases in the concentration of d-amphetamine in the brain; cardiovascular effects can be potentiated.
MAO inhibitors:
MAOI antidepressants, as well as a metabolite of furazolidone, slow amphetamine metabolism. This slowing potentiates amphetamines, increasing their effect on the release of norepinephrine and other monoamines from adrenergic nerve endings; this can cause headaches and other signs of hypertensive crisis. A variety of neurological toxic effects and malignant hyperpyrexia can occur, sometimes with fatal results.
Antihistamines:
Amphetamines may counteract the sedative effect of antihistamines.
Antihypertensives:
Amphetamines may antagonize the hypotensive effects of antihypertensives.
Chlorpromazine:
Chlorpromazine blocks dopamine and norepinephrine reuptake, thus inhibiting the central stimulant effects of amphetamines, and can be used to treat amphetamine poisoning.
Ethosuximide:
Amphetamines may delay intestinal absorption of ethosuximide.
Haloperidol:
Haloperidol blocks dopamine and norepinephrine reuptake, thus inhibiting the central stimulant effects of amphetamines.
Lithium carbonate:
The stimulatory effects of amphetamines may be inhibited by lithium carbonate.
Meperidine:
Amphetamines potentiate the analgesic effect of meperidine.
Methe nam ine therapy:
Urinary excretion of amphetamines is increased, and efficacy is reduced, by acidifying agents used in methenamine therapy.
Norepinephrine:
Amphetamines enhance the adrenergic effect of norepinephrine.
Phenobarbital:
Amphetamines may delay intestinal absorption of phenobarbital; co-administration of phenobarbital may produce a synergistic anticonvulsant action.
Phenytoin:
Amphetamines may delay intestinal absorption of phenytoin; co-administration of phenytoin may produce a synergistic anticonvulsant action.
Propoxyphene:
In cases of propoxyphene overdosage, amphetamine CNS stimulation is potentiated and fatal convulsions can occur.
Veratrum Alkaloids:
Amphetamines inhibit the hypotensive effect of veratrum alkaloids.
Drug/Laboratory Test Interactions:
.Amphetamines can cause a significant elevation in plasma corticosteroid levels. This increase is greatest in the evening.
.Amphetamines may interfere with urinary steroid determinations.
Carcinogenesis/Mutagenesis:
Mutagenicity studies and long-term studies in animals to determine the carcinogenic potential of dextroamphetamine sulfate have not been performed.
Pregnancy-Teratogenic Effects:
Pregnancy Category C:
Dextroamphetamine sulfate has been shown to have embryotoxic and teratogenic effects when administered to A/Jax mice and C57BL mice in doses approximately 41 times the maximum human dose. Embryotoxic effects were not seen in New Zealand white rabbits given the drug in doses 7 times the human dose nor in rats given 12.5 times the maximum human dose. While there are no adequate and well-controlled studies in pregnant women, there has been one report of severe congenital bony deformity, tracheoesophageal fistula, and anal atresia (Vater association) in a baby born to a woman who took dextroamphetamine sulfate with lovastatin during the first trimester of pregnancy. Dextroamphetamine sulfate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects:
Infants born to mothers dependent on amphetamines have an increased risk of premature delivery and low birth weight. Also, these infants may experience symptoms of withdrawal as demonstrated by dysphoria, including agitation, and significant lassitude.
Nursing Mothers:
Amphetamines are excreted in human milk. Mothers taking amphetamines should be advised to refrain from nursing.
Pediatric Use:
Long-term effects of amphetamines in pediatric patients have not been well established.
Amphetamines are not recommended for use in pediatric patients under 3 years of age with Attention Deficit Disorder with Hyperactivity described under INDICATIONS AND USAGE.
Clinical experience suggests that in psychotic children, administration of amphetamines may exacerbate symptoms of behavior disturbance and thought disorder.
Amphetamines have been reported to exacerbate motor and phonic tics and Tourette's syndrome. Therefore, clinical evaluation for tics and Tourette's syndrome in children and their families should precede use of stimulant medications.
Data are inadequate to determine whether chronic administration of amphetamines may be associated with growth inhibition; therefore, growth should be monitored during treatment.
Drug treatment is not indicated in all cases of Attention Deficit Disorder with Hyperactivity and should be considered only in light of the complete history and evaluation of the child. The decision to prescribe amphetamines should depend on the physician's assessment of the chronicity and severity of the child's symptoms and their appropriateness for his/her age. Prescription should not depend solely on the presence of one or more of the behavioral characteristics.
When these symptoms are associated with acute stress reactions, treatment with amphetamines is usually not indicated.
Adverse Reactions:
Cardiovascular:
Palpitations, tachycardia, elevation of blood pressure. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use.
Central Nervous System:
Psychotic episodes at recommended doses (rare), overstimulation, restlessness, dizziness, insomnia, euphoria, dyskinesia, dysphoria, tremor, headache, exacerbation of motor and phonic tics and Tourette's syndrome.
Gastrointestinal:
Dryness of the mouth, unpleasant taste, diarrhea, constipation, other gastrointestinal disturbances. Anorexia and weight loss may occur as undesirable effects.
Allergic:
Urticaria.
Endocrine:
Impotence, changes in libido.
Drug Abuse and Dependence:
Dextroamphetamine sulfate is a Schedule II controlled substance.
Amphetamines have been extensively abused. Tolerance, extreme psychological dependence and severe social disability have occurred. There are reports of patients who have increased the dosage to many times that recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG.
Manifestations of chronic intoxication with amphetamines include severe dermatoses, marked insomnia, irritability, hyperactivity and personality changes. The most severe manifestation of chronic intoxication is psychosis, often clinically indistinguishable from schizophrenia. This is rare with oral amphetamines.
Overdosage:
Individual patient response to amphetamines varies widely. While toxic symptoms occasionally occur as an idiosyncrasy at doses as low as 2 mg, they are rare with doses of less than 15 mg; 30 mg can produce severe reactions, yet doses of 400 to 500 mg are not necessarily fatal.
In rats, the oral LD50 of dextroamphetamine sulfate is 96.8 mg/kg.
Manifestations of acute overdosage with amphetamines include restlessness, tremor, hyperreflexia, rhabdomyolysis, rapid respiration, hyperpyrexia, confusion, assaultiveness, hallucinations, panic states.
Fatigue and depression usually follow the central stimulation.
Cardiovascular effects include arrhythmias, hypertension or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma.
Treatment:
Consult with a Certified Poison Control Center for up-to-date guidance and advice. Management of acute amphetamine intoxication is largely symptomatic and includes gastric lavage, administration of activated charcoal, administration of a cathartic, and sedation. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendation in this regard. Acidification of the urine increases amphetamine excretion, but is believed to increase risk of acute renal failure if myoglobinuria is present. If acute, severe hypertension complicates amphetamine overdosage, administration of intravenous phentolamine (Regitine®, CIBA) has been suggested. However, a gradual drop in blood pressure will usually result when sufficient sedation has been achieved.
Chlorpromazine antagonizes the central stimulant effects of amphetamines and can be used to treat amphetamine intoxication.
Since much of the capsule medication is coated for gradual release, therapy directed at reversing the effects of the ingested drug and at supporting the patient should be continued for as long as overdosage symptoms remain. Saline cathartics are useful for hastening the evacuation of pellets that have not already released medication.
Dosage and Administration:
Amphetamines should be administered at the lowest effective dosage and dosage should be individually adjusted. Late evening doses - particularly with the capsule form - should be avoided because of the resulting insomnia.
Narcolepsy:
Usual dose 5 to 60 mg per day in divided doses, depending on the individual patient response. Narcolepsy seldom occurs in children under 12 years of age; however, when it does, dextroamphetamine sulfate may be used. The suggested initial dose for patients aged 6 to 12 is 5 mg daily; daily dose may be raised in increments of 5 mg at weekly intervals until optimal response is obtained. In patients 12 years of age and older, start with 10 mg daily; daily dosage may be raised in increments of 10 mg at weekly intervals until optimal response is obtained. If bothersome adverse reactions appear (e.g., insomnia or anorexia), dosage should be reduced. Capsules may be used for once-a-day dosage wherever appropriate.
Attention Deficit Disorder with Hyperactivity:
Not recommended for pediatric patients under 3 years of age.
In pediatric patients 6 years of age and older, start with 5 mg once or twice daily; daily dosage may be raised in increments of 5 mg at weekly intervals until optimal response is obtained. Only in rare cases will it be necessary to exceed a total of 40 mg per day.
Capsules may be used for once-a-day dosage wherever appropriate.
Where possible, drug administration should be interrupted occasionally to determine if there is a recurrence of behavioral symptoms sufficient to require continued therapy.
HOW SUPPLIED:
Dextroamphetamine Sulfate Extended-Release Capsules are available as:
5 mg: Beige opaque cap and beige opaque body filled with white to off-white pellets. Imprinted in black ink barr 954. Available in bottles of:
30 NDC 0555-0954-01
100 NDC 0555-0954-02
10 mg: Brown opaque cap and colorless, clear body filled with white to off-white pellets. Imprinted in black ink barr 955. Available in bottles of:
30 NDC 0555-0955-01
100 NDC 0555-0955-02
15 mg: Dark brown opaque cap and colorless, clear body filled with white to off-white pellets. Imprinted in black ink barr 956. Available in bottles of:
30 NDC 0555-0956-01
100 NDC 0555-0956-02
Dispense in a tight, light-resistant container.
Store at controlled room temperature, 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [See USP].
MANUFACTURED BY
BARR LABORATORIES, INC.
POMONA, NY 10970
BR-954, 955, 956
| Dextroamphetamine Sulfate (Dextroamphetamine Sulfate) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine Sulfate (Dextroamphetamine Sulfate) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine Sulfate (Dextroamphetamine Sulfate) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Revised: 03/2006