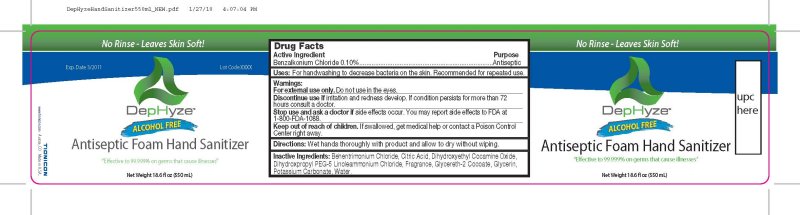
DEPHYZE ANTISEPTIC FOAM HAND SANITIZER- benzalkonium chloride spray
Artemis Bio-Solutions Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DepHyze Alcohol Free Antiseptic Foam Hand Sanitizer
Discontinue use if irritation and redness develop. If condition persists for more than 72 hours consult a doctor.
Stop use and ask a doctor if side effects occur. You may report side effects to FDA at 1-800-FDA-1088.
Keep out of reach of children. If swallowed , get medical help or contact a Poison Control Center right away.
Behentrimonium Chloride, Citric Acid, Dihydroxyethyl Cocamide Oxide, Dihydroxypropyl PEG-5 Linoleammonium Chloride, Fragrance,
Glycereth-2 Cocoate, Glycerin, Potassium Carbonate, Water.
| DEPHYZE ANTISEPTIC FOAM HAND SANITIZER
benzalkonium chloride spray |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Artemis Bio-Solutions Inc. (963442541) |
| Registrant - MicroPure Solutions, LLC dba Tionicon (805764508) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MicroPure Solutions, LLC dba Tionicon | 805764508 | manufacture(49765-200) | |