PROPULSID
-
cisapride tablet
PROPULSID
-
cisapride suspension
Ortho-McNeil-Janssen Pharmaceuticals, Inc.
----------
PROPULSID®(cisapride)
TABLETS/SUSPENSION
Rx only
Professional Package Insert
Warning:
Serious cardiac arrhythmias including ventricular tachycardia, ventricular fibrillation, torsades de pointes, and QT prolongation have been reported in patients taking PROPULSID®. From July 1993 through May 1999, more than 270 such cases have been spontaneously reported, including 70 fatalities. In approximately 85% of these cases the events occurred when PROPULSID®was used in patients with known risk factors. These risk factors included the administration of other drugs which caused QT prolongation, inhibited the cytochrome P450 3A4 enzymes that metabolize cisapride, or depleted serum electrolytes; or the presence of disorders that may have predisposed patients to arrhythmias. In approximately 0.7% of these cases, the events occurred in the absence of identified risk factors; in the remaining cases, risk factor status was unknown. Because the cases were reported voluntarily from a population of unknown size, estimates of adverse event frequency cannot be made. (See CONTRAINDICATIONS, WARNINGS, PRECAUTIONS and Drug Interactions.)
Numerous drug classes and agents increase the risk of developing serious cardiac arrhythmias. PROPULSID® is contraindicated in patients taking certain macrolide antibiotics (such as clarithromycin, erythromycin, and troleandomycin), certain antifungals (such as fluconazole, itraconazole, and ketoconazole), protease inhibitors (such as indinavir and ritonavir), phenothiazines (such as prochlorperazine and promethazine), Class IA and Class III antiarrhythmics (such as quinidine, procainamide, and sotalol); tricyclic antidepressants (such as amitriptyline); certain antidepressants (such as nefazodone and maprotiline); certain antipsychotic medications (such as sertindole), as well as other agents (such as bepridil, sparfloxacin, and grapefruit juice). (See PRECAUTIONS: Drug Interactions.) The preceding list is not comprehensive.
QT prolongation, torsades de pointes (sometimes with syncope), cardiac arrest and sudden death have been reported in patients taking PROPULSID®without the above-mentioned contraindicated drugs. Most patients had disorders that may have predisposed them to arrhythmias with PROPULSID®. These include history of prolonged electrocardiographic QT intervals or known family history of congenital long QT syndrome; history of ventricular arrhythmias, ischemic or valvular heart disease; other structural heart defects; cardiomyopathy; congestive heart failure; clinically significant bradycardia; sinus node dysfunction; second or third degree atrioventricular block; respiratory failure; or conditions that result in electrolyte disorders (hypokalemia, hypocalcemia, and hypomagnesemia), such as severe dehydration, vomiting, or malnutrition; eating disorders; renal failure; or the administration of potassium-wasting diuretics or insulin in acute settings. PROPULSID® is contraindicated in patients with these conditions.
A 12-lead ECG should be performed prior to administration of PROPULSID®. Treatment with PROPULSID® should not be initiated if the QTc value exceeds 450 milliseconds. Serum electrolytes (potassium, calcium, and magnesium) and creatinine should be assessed prior to administration of PROPULSID® and whenever conditions develop that may affect electrolyte balance or renal function. (See DOSAGE AND ADMINISTRATION.)
If syncope, rapid or irregular heartbeat develop, patients should immediately stop taking PROPULSID® and seek the attention of a physician.
Recommended doses of PROPULSID®should not be exceeded.
DESCRIPTION
PROPULSID® (cisapride) Tablets and Suspension contain cisapride as the monohydrate, which is an oral gastrointestinal agent chemically designated as (±)-cis-4-amino-5-chloro-N-[1-[3-(4-fluorophenoxy)propyl]-3-methoxy-4-piperidinyl]-2-methoxybenzamide monohydrate. Its empirical formula is C23H29ClFN3O4•H2O. The molecular weight is 483.97 and the structural formula is: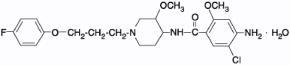
Cisapride as the monohydrate is a white to slightly beige odorless powder. It is practically insoluble in water, sparingly soluble in methanol, and soluble in acetone. Each 1.04 mg of cisapride as the monohydrate is equivalent to one mg of cisapride.
PROPULSID® is available for oral use in tablets containing cisapride as the monohydrate equivalent to 10 mg or 20 mg of cisapride and as a suspension containing the equivalent of 1 mg/mL of cisapride. The inactive ingredients in the tablets are colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, povidone, and starch (corn). The 20 mg tablets also contain FD&C Blue No. 2 aluminum lake. The inactive ingredients in the suspension are hydroxypropyl methylcellulose, methylparaben, microcrystalline cellulose and carboxymethylcellulose sodium, polysorbate 20, propylparaben, sodium chloride, sorbitol, and water. The 1 mg/mL suspension also contains artificial cherry cream flavor and FD&C Red No. 40.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Cisapride is metabolized mainly via the cytochrome P450 3A4 enzyme. PROPULSID® (cisapride) is extensively metabolized; unchanged drug accounts for less than 10% of urinary and fecal recovery following oral administration. Norcisapride, formed by N-dealkylation, is the principal metabolite in plasma, feces and urine. PROPULSID® is rapidly absorbed after oral administration; peak plasma concentrations are reached 1 to 1.5 hours after dosing. The absolute bioavailability of PROPULSID® is 35-40%. When gastric acidity was reduced by high dose histamine H2 receptor blocker and sodium bicarbonate in fasting subjects, there was a decrease in the rate, and to a lesser degree the extent, of PROPULSID® tablet absorption. (This has not been established for the suspension.) PROPULSID® binds to an extent of 97.5-98% to plasma proteins, mainly to albumin. The volume of distribution of PROPULSID® is about 180 L, indicating extensive tissue distribution.
The plasma clearance of PROPULSID® is about 100 mL/min. The mean terminal half-life reported for PROPULSID® ranges from 6 to 12 hours; longer half-lives, up to 20 hours, have been reported following intravenous (IV) administration.
There was no unusual drug accumulation due to time-dependent or non-linear changes in pharmacokinetics. After cessation of the repeated dosing, the elimination half-lives (8 to 10 hr) were in the same order as after single dosing. The degree of accumulation of PROPULSID® and/or its metabolites may be somewhat higher in patients with hepatic or renal impairment and in elderly patients compared to young healthy volunteers, but the differences are not consistent. Dose adjustments are recommended in patients with hepatic impairment. (See DOSAGE AND ADMINISTRATION.)
The pharmacokinetics of cisapride in pediatric patients are not well characterized. Therefore, it is unknown if the dose-response relationship in the adult population can be extrapolated to the pediatric population. (See PRECAUTIONS: Pediatric Use.)
Pharmacodynamics
The onset of pharmacological action of cisapride is approximately 30 to 60 minutes after oral administration.
Cisapride promotes gastric motility. The mechanism of action of cisapride is thought to be primarily enhancement of release of acetylcholine at the myenteric plexus. Cisapride does not induce muscarinic or nicotinic receptor stimulation, nor does it inhibit acetylcholinesterase activity. It is less potent than metoclopramide in dopamine receptor-blocking effects in rats. It does not increase or decrease basal or pentagastrin-induced gastric acid secretion.
In vitro studies have shown that cisapride is a serotonin-4 (5-HT4) receptor agonist.
Electrophysiological studies in in vivo anesthetized guinea pig and rabbit models and in vitro isolated rabbit Purkinje fibers and ventricular papillary muscle and isolated rabbit ventricular myocyte models, have shown that cisapride prolonged cardiac repolarization without slowing conduction by selectively blocking the rapid component of the delayed rectifying K+ current (Ikr) which leads to a lengthening of the action potential (QT Syndrome).
Esophagus:
Twenty milligrams oral cisapride given once to healthy volunteers increased lower esophageal sphincter pressure (LESP), starting 45 minutes after dosing, with a peak response at 75 minutes. The full duration of the effect was not monitored, and doses smaller than 20 mg were ineffective. Ten milligrams oral cisapride, administered 3 times daily for several days to patients with gastroesophageal reflux disease (GERD), resulted in a significant increase in LESP, and an increased esophageal acid clearance.
Stomach:
Cisapride (single 10 mg doses or 10 mg given orally 3 times daily up to six weeks) significantly accelerated gastric emptying of both liquids and solids. Acceleration of gastric emptying, measured over a four hour period following a radio-labeled test meal given at lunch time, was greatest when 10 mg cisapride was given both in the morning and again before the test meal, intermediate when 20 mg was given as a single administration in the morning and least when only 10 mg was given on the morning of the test meal. The increases in gastric emptying were proportional to the plasma levels of cisapride measured in these subjects over the same 4 hours that the gastric emptying test was conducted.
Clinical Trials
Clinical trials have shown that cisapride can reduce the severity of symptoms of nocturnal heartburn associated with gastroesophageal reflux disease. Two placebo-controlled studies, one using a dose of 10 mg q.i.d., the other both 10 and 20 mg q.i.d., showed effects on nighttime heartburn, although the 10 mg dose in the second study was only marginally effective. There were no consistent effects on daytime heartburn, symptoms of regurgitation, or histopathology of the esophagus. Use of antacids was only infrequently affected and slightly decreased. In a third controlled trial of similar design to the others, neither 10 mg nor 20 mg taken 4 times daily was superior to placebo. In these clinical trials cisapride did not show a significant effect on LESP.
In a clinical trial comparing 10 mg cisapride to placebo, pH probe evaluation, in a relatively small number of patients, did not reveal a significant difference in pH.
INDICATIONS AND USAGE
PROPULSID® (cisapride) is indicated for the symptomatic treatment of adult patients with nocturnal heartburn due to gastroesophageal reflux disease. Because of the risk of serious, and sometimes fatal, ventricular arrhythmias (see Boxed Warning), PROPULSID® should generally be reserved for patients who do not respond adequately to lifestyle modifications (See PRECAUTIONS: Information for Patients and Medication Guide), antacids and gastric acid reducing agents.
CONTRAINDICATIONS
Serious cardiac arrhythmias including ventricular tachycardia, ventricular fibrillation, torsades de pointes, and QT prolongation have been reported in patients taking PROPULSID®(cisapride) with other drugs that inhibit cytochrome P450 3A4 or that prolong the QT interval. Some of these events have been fatal. Concomitant oral or intravenous administration of these drugs with PROPULSID®is contraindicated. PROPULSID®is also contraindicated for patients with disorders that may predispose them to arrhythmias. (See Boxed Warning, WARNINGS, PRECAUTIONS and Drug Interactions.)
PROPULSID® should not be used in patients in whom an increase in gastrointestinal motility could be harmful, e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation.
PROPULSID® is contraindicated in patients with known sensitivity or intolerance to the drug.
WARNINGS
PROPULSID® (cisapride) undergoes metabolism mainly by the hepatic cytochrome P450 3A4 isoenzyme. Drugs which inhibit this enzyme can lead to elevated cisapride blood levels. (See PRECAUTIONS and Drug Interactions.)
Numerous cases of serious cardiac arrhythmias, including ventricular arrhythmias and torsades de pointes associated with QT prolongation, have been reported in patients taking PROPULSID® alone or with the drugs listed above, or with disorders that may have predisposed them to arrhythmias. Some of these patients did not have cardiac disease; however, most had been receiving multiple other medications and had pre-existing cardiac disease or risk factors for arrhythmias. Some of these cases have been fatal. (See Boxed Warning.)
PRECAUTIONS
General:
Potential benefits should be weighed against risks prior to administration of PROPULSID® (cisapride) to patients who have conditions that could predispose them to the development of serious arrhythmias, such as multiple organ failure, COPD, apnea and advanced cancer. (See CONTRAINDICATIONS.)
Information for Patients:
Patients should be warned against concomitant use of promethazine (Phenergan®), bepridil (Vascor®), quinidine (such as Quinidex®, Cardioquin®, Quinaglute®), procainamide (Procanbid®), sotalol (Betapace®), erythromycin (such as E.E.S.®, E-Mycin®, Ilotycin®, Pediazole®), clarithromycin (Biaxin®), troleandomycin (TAO®), sparfloxacin (Zagam®), amitriptyline (Elavil®), maprotiline (Ludiomil®), nefazodone (Serzone®), fluconazole (Diflucan®), itraconazole (Sporanox®), ketoconazole (Nizoral®), prochlorperazine (Compazine®), sertindole, indinavir (Crixivan®), ritonavir (Norvir®) and warfarin (Coumadin®). (See Drug Interactions.) The preceding list is not comprehensive.
Recommended doses should not be exceeded.
Patients should be advised to stop PROPULSID® and seek medical attention if they faint or become faint, dizzy, experience an irregular heartbeat or pulse, or any other unusual symptoms while using PROPULSID®.
Patients should be questioned about concomitant medication use. Patients taking PROPULSID® should also be advised to inform their physician when new medications are prescribed.
Patients should be advised to refrain from consuming grapefruit juice for the duration of their PROPULSID® therapy.
Although PROPULSID® does not affect psychomotor function nor does it induce sedation or drowsiness when used alone, patients should be advised that the sedative effects of benzodiazepines and of alcohol may be enhanced by PROPULSID®.
Patients should be advised that generally the following lifestyle changes should be tried before using any drug for nighttime heartburn, including PROPULSID®: avoiding alcohol, quitting/decreasing cigarette smoking, elevating the head of the bed, avoiding large meals/meals just before bedtime, losing weight, avoiding fatty foods, chocolate, caffeine, or citrus.
Patients should be given the Medication Guide for additional information.
Drug Interactions:
Cisapride is metabolized mainly via the cytochrome P450 3A4 enzyme. In some cases where serious ventricular arrhythmias, QT prolongation, and torsades de pointes have occurred when PROPULSID® was taken in conjunction with one of the cytochrome P450 3A4 inhibitors, elevated blood cisapride levels were noted at the time of the QT prolongation.
Antibiotics:
In vitro and/or in vivo data show that clarithromycin, erythromycin and troleandomycin markedly inhibit the metabolism of PROPULSID®, which can result in an increase in plasma cisapride levels and prolongation of the QT interval on the ECG.
Anticholinergics:
Concurrent administration of certain anticholinergic compounds, such as belladonna alkaloids and dicyclomine, would be expected to compromise the beneficial effects of PROPULSID®.
Anticoagulants (oral):
In patients receiving oral anticoagulants, the coagulation times were increased in some cases. It is advisable to check coagulation time within the first few days after the start and discontinuation of PROPULSID® therapy, with an appropriate adjustment of the anticoagulant dose, if necessary.
Antidepressants:
In vitro data indicate that nefazodone inhibits the metabolism of PROPULSID®, which can result in an increase in plasma cisapride levels and prolongation of the QT interval on the ECG.
Antifungals:
In vitro and/or in vivo data indicate that fluconazole, itraconazole and oral ketoconazole markedly inhibit the metabolism of PROPULSID®, which can result in an increase in plasma cisapride levels and prolongation of the QT interval on the ECG. Human pharmacokinetic data indicate that oral ketoconazole markedly inhibits the metabolism of cisapride, resulting in a mean eight-fold increase in AUC of cisapride. A study in 14 normal male and female volunteers suggests that coadministration of PROPULSID® and ketoconazole can result in prolongation of the QT interval on the ECG.
Diuretics:
Drugs such as furosemide and the thiazides are associated with depletion of electrolytes which may result in PROPULSID®-induced cardiac arrhythmias. Serum electrolytes should be assessed in diuretic-treated patients before initiating PROPULSID® therapy and periodically thereafter. PROPULSID®-treated patients to whom diuretic therapy is added should undergo careful electrolyte monitoring after diuretic initiation.
H2 receptor antagonists:
Cimetidine coadministration leads to an increased peak plasma concentration and AUC of PROPULSID®; there is no effect on PROPULSID® absorption when it is coadministered with ranitidine. The gastrointestinal absorption of cimetidine and ranitidine is accelerated when they are coadministered with PROPULSID®.
Protease inhibitors:
In vitro data indicate that indinavir and ritonavir markedly inhibit the metabolism of PROPULSID® which can result in an increase in plasma cisapride levels and prolongation of the QT interval on the ECG.
Other:
Co-administration of grapefruit juice with PROPULSID® increases the bioavailability of cisapride by an average of 50%. Patients on PROPULSID® should refrain from consuming grapefruit juice for the duration of their PROPULSID® therapy.
PROPULSID® should not be used concomitantly with other drugs known to prolong the QT interval: certain antiarrhythmics, including those of Class IA (such as quinidine and procainamide) and Class III (such as sotalol); tricyclic antidepressants (such as amitriptyline); certain tetracyclic antidepressants (such as maprotiline); certain antipsychotic medications (such as sertindole); bepridil, and sparfloxacin. The preceding lists are not comprehensive.
The acceleration of gastric emptying by PROPULSID® could affect the rate of absorption of other drugs. Patients receiving narrow therapeutic ratio drugs or other drugs that require careful titration should be followed closely; if plasma levels are being monitored, they should be reassessed.
Carcinogenesis, mutagenesis, impairment of fertility:
In a twenty-five month oral carcinogenicity study in rats, cisapride at daily doses up to 80 mg/kg was not tumorigenic. For a 50 kg person of average height (1.46 m2 body surface area), this dose represents 50 times the maximum recommended human dose (1.6 mg/kg/day) on a mg/kg basis and 7 times the maximum recommended human dose (54.4 mg/m2) on a body surface area basis. In a nineteen month oral carcinogenicity study in mice, cisapride at daily doses up to 80 mg/kg was not tumorigenic. This dose represents 50 times the maximum recommended human dose on a mg/kg basis and about 4 times the maximum recommended human dose on a body surface area basis.
Cisapride was not mutagenic in the in vitro Ames test, human lymphocyte chromosomal aberration test, mouse lymphoma cell forward mutation test, and rat hepatocyte UDS test and in vivo rat micronucleus test, male and female mouse dominant lethal mutations tests, and sex linked recessive lethal test in male Drosophila melanogaster.
Fertility and reproductive performance studies were conducted in male and female rats. Cisapride was found to have no effect on fertility and reproductive performance of male rats at oral doses up to 160 mg/kg/day (100 times the maximum recommended human dose on a mg/kg basis and 14 times the maximum recommended human dose on a mg/m2 basis). In the female rats, cisapride at oral doses of 40 mg/kg/day and higher prolonged the breeding interval required for impregnation. Similar effects were also observed at maturity in the female offspring (F1) of the female rats (F0) treated with oral doses of cisapride at 10 mg/kg/day or higher. Cisapride at an oral dose of 160 mg/kg/day also exerted contragestational/pregnancy disrupting effects in female rats (F0).
Pregnancy: Teratogenic effects: Pregnancy category C:
Oral teratology studies have been conducted in rats (doses up to 160 mg/kg/day) and rabbits (doses up to 40 mg/kg/day). There was no evidence of a teratogenic potential of cisapride in rats or rabbits. Cisapride was embryotoxic and fetotoxic in rats at a dose of 160 mg/kg/day (100 times the maximum recommended human dose on a mg/kg basis and 14 times the maximum recommended human dose on a mg/m2 basis) and in rabbits at a dose of 20 mg/kg/day (approximately 12 times the maximum recommended human dose on a mg/kg basis) or higher. It also produced reduced birth weights of pups in rats at 40 and 160 mg/kg/day and adversely affected the pup survival. There are no adequate and well-controlled studies in pregnant women. Cisapride should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the mother and the fetus.
Nursing Mothers:
Cisapride is excreted in human milk at concentrations approximately one twentieth of those observed in plasma. Caution should be exercised when PROPULSID® is administered to a nursing woman, and particular care must be taken if the nursing infant or the mother is taking a drug that might alter PROPULSID®'s metabolism in the infant. (See CONTRAINDICATIONS, WARNINGS, PRECAUTIONS and Drug Interactions.)
Pediatric Use:
Safety and effectiveness in pediatric patients under the age of 16 years have not been established for any indication. Although causality has not been established, serious adverse events, including death, have been reported in infants and children treated with PROPULSID®. Several pediatric deaths were due to cardiovascular events (third degree heart block and ventricular tachycardia). Pediatric deaths have been associated with seizures and there has been at least one case of “sudden unexplained death” in a 3-month-old infant. Other unlabeled potentially serious events which have been reported in pediatric patients include: antinuclear antibody (ANA) positive, anemia, hemolytic anemia, methemoglobinemia, hyperglycemia, hypoglycemia with acidosis, unexplained apneic episodes, confusion, impaired concentration, depression, apathy, visual changes accompanied by amnesia, and severe photosensitivity reaction. (See OVERDOSAGE.)
Geriatric Use:
Steady-state plasma levels are generally higher in older than in younger patients, due to a moderate prolongation of the elimination half-life. Therapeutic doses, however, are similar to those used in younger adults.
The rate of common adverse experiences in patients greater than 65 years of age in clinical trials was similar to that in younger adults.
ADVERSE REACTIONS
In the U.S. clinical trial population of 1728 patients (comprising 506 with gastroesophageal reflux disorders, and the remainder with other disorders) the following adverse experiences were reported in more than 1% of patients treated with PROPULSID® (cisapride) and at least as often on PROPULSID® as on placebo.
| System/Adverse Event | PROPULSID®
N=1042 | Placebo N=686 |
| Central & Peripheral Nervous Systems | ||
| Headache | 19.3% | 17.1% |
| Gastrointestinal | ||
| Diarrhea | 14.2 | 10.3 |
| Abdominal pain | 10.2 | 7.7 |
| Nausea | 7.6 | 7.6 |
| Constipation | 6.7 | 3.4 |
| Flatulence | 3.5 | 3.1 |
| Dyspepsia | 2.7 | 1.0 |
| Respiratory System | ||
| Rhinitis | 7.3 | 5.7 |
| Sinusitis | 3.6 | 3.5 |
| Coughing | 1.5 | 1.2 |
| Resistance Mechanism | ||
| Viral infection | 3.6 | 3.2 |
| Upper respiratory tract infection | 3.1 | 2.8 |
| Body as a Whole | ||
| Pain | 3.4 | 2.3 |
| Fever | 2.2 | 1.5 |
| Urinary System | ||
| Urinary tract infection | 2.4 | 1.9 |
| Micturition frequency | 1.2 | 0.6 |
| Psychiatric | ||
| Insomnia | 1.9 | 1.3 |
| Anxiety | 1.4 | 1.0 |
| Nervousness | 1.4 | 0.7 |
| Skin & Appendages | ||
| Rash | 1.6 | 1.6 |
| Pruritus | 1.2 | 1.0 |
| Musculoskeletal System | ||
| Arthralgia | 1.4 | 1.2 |
| Vision | ||
| Abnormal vision | 1.4 | 0.3 |
| Reproductive, Female | ||
| Vaginitis | 1.2 | 0.9 |
The following adverse events also reported in more than 1% of PROPULSID® patients were more frequently reported on placebo: dizziness, vomiting, pharyngitis, chest pain, fatigue, back pain, depression, dehydration and myalgia.
Diarrhea, abdominal pain, constipation, flatulence and rhinitis all occurred more frequently in patients using 20 mg of PROPULSID® than in patients using 10 mg.
Additional adverse experiences reported to occur in 1% or less of patients in the U.S. clinical studies are: dry mouth, somnolence, palpitation, migraine, tremor and edema.
In other U.S. and international trials and in postmarketing experience, there have been rare reports of seizures and extrapyramidal effects. Also reported have been tachycardia, elevated liver enzymes, hepatitis, thrombocytopenia, leukopenia, aplastic anemia, pancytopenia and granulocytopenia. The relationship of PROPULSID® to the event was not clear in these cases.
Cardiac arrhythmias, including ventricular tachycardia, ventricular fibrillation, torsades de pointes, and QT prolongation, in some cases resulting in death, have been reported. (See CONTRAINDICATIONS, WARNINGS, PRECAUTIONS and Drug Interactions.)
Ongoing Postmarketing Surveillance: Serious cardiac arrhythmias including ventricular tachycardia, ventricular fibrillation, torsades de pointes, and QT prolongation have been reported in patients taking PROPULSID®. From July 1993 through May 1999, more than 270 such cases have been spontaneously reported, including 70 fatalities. In approximately 85% of these cases the events occurred when PROPULSID®was used in patients with known risk factors. These risk factors included the administration of other drugs which caused QT prolongation, inhibited the cytochrome P450 3A4 enzymes that metabolize cisapride, or depleted serum electrolytes; or the presence of disorders that may have predisposed patients to arrhythmias. In approximately 0.7% of these cases, the events occurred in the absence of identified risk factors; in the remaining cases, risk factor status was unknown. Because the cases were reported voluntarily from a population of unknown size, estimates of adverse event frequency cannot be made. (See Boxed Warning, CONTRAINDICATIONS, WARNINGS, PRECAUTIONS and Drug Interactions.) PROPULSID®-induced serious ventricular arrhythmias and death may not correlate with the degree of drug-induced prolongation of the QT interval detected by 12-lead ECG.
In addition to the cardiovascular adverse events, the following events have been identified during post-approval use of PROPULSID® in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion in this insert due to a combination of their seriousness, frequency of reporting, or potential causal connection to PROPULSID®: allergic reactions, including bronchospasm, urticaria, and angioedema; possible exacerbation of asthma; psychiatric events, including confusion, depression, suicide attempt, and hallucinations; extrapyramidal effects including akathisia, Parkinson-like symptoms, dyskinetic and dystonic reactions; gynecomastia, female breast enlargement, urinary incontinence, hyperprolactinemia and galactorrhea.
The following events were specifically reported in the pediatric population: antinuclear antibody (ANA) positive, anemia, hemolytic anemia, methemoglobinemia, hyperglycemia, hypoglycemia with acidosis, unexplained apneic episodes, confusion, impaired concentration, depression, apathy, visual changes accompanied by amnesia, and severe photosensitivity reaction.
There have been rare cases of sinus tachycardia reported. Rechallenge precipitated the tachycardia again in some of those patients.
OVERDOSAGE
With overdose, rare cases of QT prolongation and ventricular arrhythmia have been reported.
A one-month-old male infant received 2 mg/kg of cisapride four times per day for 5 days. The patient developed third degree heart block and subsequently died of right ventricular perforation caused by pacemaker wire insertion.
In instances of overdose, patients should be evaluated for possible QT prolongation and ventricular arrhythmias, including torsades de pointes. Treatment should include gastric lavage and/or activated charcoal, close observation and general supportive measures.
Reports of overdosage with PROPULSID® (cisapride) also include an adult who took 540 mg and for 2 hours experienced retching, borborygmi, flatulence, stool frequency and urinary frequency.
Single oral doses of cisapride at 4000 mg/kg, 160 mg/kg, 1280 mg/kg and 640 mg/kg were lethal in adult rats, neonatal rats, mice, and dogs, respectively. Symptoms of acute toxicity were ptosis, tremors, convulsions, dyspnea, loss of righting reflex, catalepsy, catatonia, hypotonia and diarrhea.
DOSAGE AND ADMINISTRATION
5 mL (1 teaspoon) suspension = 5 mg.
A 12-lead ECG should be performed prior to administration of PROPULSID® (cisapride). Treatment with PROPULSID® should not be initiated if the QTc value exceeds 450 milliseconds. Serum electrolytes (potassium, calcium, and magnesium) and creatinine should be assessed prior to administration of PROPULSID® and whenever conditions develop that may affect electrolyte balance or renal function.
Adults: Initiate therapy with one 10 mg tablet of PROPULSID® or 10 mL of the suspension 4 times daily at least 15 minutes before meals and at bedtime. In some patients the dosage will need to be increased to 20 mg, given as above, to obtain a satisfactory result.
Caution must be exercised in elderly patients since there is a significant proportion who have conditions or use other drugs which contraindicate the use of PROPULSID®. A 12-lead ECG and serum electrolyte measurement should be performed prior to treatment with PROPULSID®. In elderly patients, steady-state plasma levels are generally higher due to a moderate prolongation of the elimination half-life. Therapeutic doses, however, are similar to those used in younger adults.
It is recommended that the daily dose be halved in patients with hepatic insufficiency.
The minimum effective dose of PROPULSID® should be used. Recommended doses should not be exceeded. PROPULSID® should be discontinued if relief of nocturnal heartburn does not occur.
HOW SUPPLIED
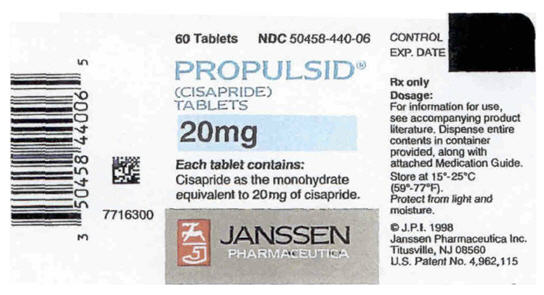
PROPULSID® (cisapride) Tablets are provided as scored white tablets debossed "Janssen" and P/10 containing the equivalent of 10 mg of cisapride in blister packages of 100 (NDC 50458-430-01) and in unit of use bottles of 120 (NDC 50458-430-12). PROPULSID® is also provided as blue tablets, debossed "Janssen" and P/20, containing the equivalent of 20 mg cisapride in blister packages of 100 (NDC 50458-440-01) and in unit of use bottles of 60 (NDC 50458-440-06).
PROPULSID® Suspension is provided as a bright pink homogeneous suspension containing the equivalent of 1 mg/mL of cisapride in 16 oz. unit of use bottles containing 450 mL (NDC 50458-450-45).
Unit of use bottles should be dispensed as an intact unit. The Medication Guide should be dispensed with the product.
Store at 15°-25°C (59°-77°F). Protect the tablets from moisture. The 20 mg tablets should also be protected from light.
Phenergan is a registered trademark of Wyeth-Ayerst Laboratories
Vascor is a registered trademark of Ortho-McNeil Pharmaceutical
Quinidex is a registered trademark of A. H. Robins Co., Inc.
Cardioquin is a registered trademark of Purdue Frederick
Quinaglute and Betapace are registered trademarks of Berlex Laboratories
Procanbid is a registered trademark of Monarch Pharmaceuticals
E.E.S., Biaxin and Norvir are registered trademarks of Abbott Laboratories
E-Mycin is a registered trademark of Knoll Laboratories
Ilotycin is a registered trademark of Dista Products Company
Pediazole is a registered trademark of Ross Products Division
TAO and Diflucan are registered trademarks of Pfizer, Inc.
Zagam is a registered trademark of Rhone-Poulenc Rorer Pharmaceuticals
Elavil is a registered trademark of Zeneca Pharmaceuticals
Ludiomil is a registered trademark of Novartis Pharmaceuticals Corporation
Serzone is a registered trademark of Bristol-Myers Squibb Co.
Compazine is a registered trademark of SmithKline Beecham Pharmaceuticals
Crixivan is a registered trademark of Merck & Co., Inc.
JANSSEN PHARMACEUTICA
Titusville, New Jersey 08560
7502617
U.S. Patent No. 4,962,115
Revised May 1999, January 2000
©JPPLP 2000
MEDICATION GUIDE
IMPORTANT: READ COMPLETELY BEFORE USE. Do not take PROPULSID® if you have a medical condition or take a drug listed in this Medication Guide in the section “Who Should Not Take PROPULSID®?”
MEDICATION GUIDE
Brand Name: PROPULSID® (pro-pul-sid)
Generic Name: cisapride
Available as: Tablets and Suspension (liquid form)
What is the Most Important Information I Should Know About PROPULSID® (cisapride)?
PROPULSID® may cause serious irregular heartbeats that may cause death. Taking PROPULSID® together with certain other medicines or if you have certain medical conditions increases the chance that you will have irregular heartbeats. PROPULSID® should never be taken with these other medicines or if you have these conditions. A list of these medicines and these medical conditions is in the section “Who Should Not Take PROPULSID®?”. If you faint or feel faint, become dizzy or have irregular heartbeats while using PROPULSID®, stop taking PROPULSID® and get medical help right away.
What is PROPULSID® (cisapride)?
PROPULSID® is a medicine approved only to treat the symptoms of nighttime heartburn in adults. Nocturnal, or nighttime, heartburn is a common symptom of a medical condition called gastroesophageal reflux disease (GERD). It occurs when stomach contents wash back, or “reflux,” into the esophagus (a muscular tube that carries food from the mouth to the stomach). Reflux is very common at nighttime because stomach contents can easily wash backwards when you are lying down. Usually, physicians recommend that patients with nighttime heartburn make simple lifestyle changes and use antacids or acid-reducing agents to relieve their symptoms. (See the section “What Else Can I Do for Nighttime Heartburn?” for more details.) These other lifestyle changes and medicines should be tried first because of the risk of serious, and sometimes fatal, irregular heartbeats associated with the use of PROPULSID®.
Who Should Not Take PROPULSID® (cisapride)?
Some patients who have taken certain medicines together with PROPULSID® have experienced serious problems such as fainting, dizziness and irregular heartbeats. These problems can cause death. Medications that should never be taken with PROPULSID® include:
| Type of Drug | Examples of Generic Names (Brand Name) |
| Anti-allergy: | promethazine (Phenergan®) |
| Anti-angina: | bepridil (Vascor®) |
| (for heart pain) | |
| Antiarrhythmics: | quinidine (such as Quinidex®, Cardioquin®, Quinaglute®) |
| (for irregular | procainamide (Procanbid®) |
| heart rhythm) | sotalol (Betapace®) |
| Antibiotics: | erythromycin (such as E.E.S.®, E-Mycin®, Ilotycin®, Pediazole®) |
| clarithromycin (Biaxin®), troleandomycin (TAO®) | |
| sparfloxacin (Zagam®) | |
| Antidepressants: | amitriptyline (Elavil®) |
| maprotiline (Ludiomil®) | |
| nefazodone (Serzone®) | |
| Antifungals: | fluconazole (Diflucan®) |
| itraconazole (Sporanox®) | |
| oral ketoconazole (Nizoral®) | |
| Anti-nausea: | prochlorperazine (Compazine®) |
| promethazine (Phenergan®) | |
| Antipsychotics: | sertindole |
| prochlorperazine (Compazine®) | |
| Protease Inhibitors: | indinavir (Crixivan®) |
| ritonavir (Norvir®) |
- This is not a complete list of medications that you should not take. Therefore, tell your doctor about all other prescriptions and nonprescription drugs you are taking, including herbal supplements. While taking PROPULSID®, do not start a new medicine without first consulting your doctor or pharmacist.
Also, you should not take PROPULSID® if you have any of these conditions:
- a history of irregular heartbeats
- an abnormal electrocardiogram (ECG or EKG)
- heart disease
- kidney disease
- lung disease
- low blood levels of potassium, calcium or magnesium
- an eating disorder (such as bulimia or anorexia)
- your body has suddenly lost a lot of water
- persistent vomiting
- Tell your doctor if you have any type of medical condition, especially a heart condition or kidney or lung disease. Be sure your doctor knows about both your personal and family medical history before you take PROPULSID®.
- If you have not tried other medicines to relieve your nighttime heartburn, tell your doctor before using PROPULSID®.
- The safety and effectiveness of PROPULSID® in children younger than 16 years have not been demonstrated for any use. Serious adverse events, including death, have been reported in infants and children while being treated with PROPULSID®, although there is no clear evidence that PROPULSID® caused them.
How Should I Take PROPULSID® (cisapride)?
- Take PROPULSID® exactly as your doctor prescribes it.
- Never take more than the recommended dose of PROPULSID®. Always take your medicine for as long as the doctor has prescribed it, even if you are beginning to feel better right away.
- If you forget to take a dose, do not take the missed dose. Take the next dose at the regularly scheduled time. Never take more than your prescribed dose at any one time to make up for the missed dose.
- PROPULSID® does not work for everyone. If you do not get relief of your nighttime heartburn, talk to your doctor about whether to stop using PROPULSID®.
What Should I Avoid While Taking PROPULSID® (cisapride)?
- Never take PROPULSID® with the medications listed in the above section Who Should Not Take PROPULSID® (cisapride)?
- Do not take prescription or non-prescription medicines while taking PROPULSID® without checking first with your doctor.
- Do not drink grapefruit juice at any time while you are on PROPULSID® therapy. Drinking grapefruit juice while you are on PROPULSID® therapy may cause the same kinds of serious irregular heartbeats as taking PROPULSID® with the wrong medicines.
- Tell your doctor if you are pregnant or nursing. Your doctor will talk to you about whether you should take PROPULSID® while pregnant or nursing, based on the benefits and risks.
What are the Possible Side Effects of PROPULSID® (cisapride)?
- PROPULSID® may cause serious irregular heartbeats that may cause death.
Stop taking PROPULSID® and call your doctor right away if you
- faint or feel faint
- become dizzy
- have irregular heartbeats or pulse
- develop any unusual symptoms
These may be signs of a serious side effect.
- PROPULSID's® most common side effects are headache, diarrhea, stomach pain, nausea, constipation and a runny nose. Other side effects have been reported less often. Be sure to ask your doctor or pharmacist about the side effects of any medicine that you are taking, including PROPULSID®.
- PROPULSID® may cause you to become more drowsy than normal if you drink alcohol or take medicines called benzodiazepines (used to reduce anxiety).
What Else Can I do for Nighttime Heartburn?
- Stop smoking or reduce the number of cigarettes you smoke.
- Elevate the head of your bed when you sleep.
- Avoid eating large meals or eating just before bedtime.
- Do not lie down right after eating.
- If you are overweight or your doctor recommends it, try to lose weight.
- Avoid fatty foods and foods containing chocolate, caffeine or citrus.
- Avoid alcoholic drinks.
All of these lifestyle changes will help to restore your body to a more normal way of functioning.
General Information
This leaflet provides a summary of information about PROPULSID®. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you need to report problems associated with the use of PROPULSID®, contact your doctor. If you have additional questions or concerns, or want more information about PROPULSID®, contact your doctor or pharmacist. You can also call the Janssen One-to-One™ Customer Action Center toll-free at 1-800-526-7736 for more information. Your pharmacist also has a longer leaflet about PROPULSID® that is written for health professionals that you can ask to read. This medication was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Phenergan is a registered trademark of Wyeth-Ayerst Laboratories
Vascor is a registered trademark of Ortho-McNeil Pharmaceutical
Quinidex is a registered trademark of A. H. Robins Co., Inc.
Cardioquin is a registered trademark of Purdue Frederick
Quinaglute and Betapace are registered trademarks of Berlex Laboratories
Procanbid is a registered trademark of Monarch Pharmaceuticals
E.E.S., Biaxin and Norvir are registered trademarks of Abbott Laboratories
E-Mycin is a registered trademark of Knoll Laboratories
Ilotycin is a registered trademark of Dista Products Company
Pediazole is a registered trademark of Ross Products Division
TAO and Diflucan are registered trademarks of Pfizer, Inc.
Zagam is a registered trademark of Rhone-Poulenc Rorer Pharmaceuticals
Elavil is a registered trademark of Zeneca Pharmaceuticals
Ludiomil is a registered trademark of Novartis Pharmaceuticals Corporation
Serzone is a registered trademark of Bristol-Myers Squibb Co.
Compazine is a registered trademark of SmithKline Beecham Pharmaceuticals
Crixivan is a registered trademark of Merck & Co., Inc.
7515202
U.S. Patent No. 4,962,115
May 1999, January 2000
© JPPLP 2000
Janssen Pharmaceutica
Titusville, NJ 08560
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle
60 Tablets
NDC 50458-440-06
PROPULSID®
(CISAPRIDE)
TABLETS
20mg
Each tablet contains:
Cisapride as the monohydrate
equivalent to 20mg of cisapride.
JANSSEN
PHARMACEUTICA
| PROPULSID
cisapride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA020210 | 07/01/1993 | 07/01/2000 |
| PROPULSID
cisapride tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA020210 | 07/01/1993 | 07/01/2000 |
| PROPULSID
cisapride suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA020398 | 09/01/1995 | 07/01/2000 |
| Labeler - Ortho-McNeil-Janssen Pharmaceuticals, Inc. (063137772) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Janssen Pharmaceutica, NV | 374747970 | API MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Janssen Pharmaceutica | 989673884 | API MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Janssen Pharmaceutica, NV | 370005019 | ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Janssen Ortho LLC | 062191882 | MANUFACTURE, ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ortho-McNeil-Janssen Pharmaceuticals, Inc | 063137772 | ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Centocor Ortho Biotech Products, L.P. | 804684207 | ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Catalent Pharma Solutions, LLC | 806746405 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ortho-McNeil-Janssen Pharmaceuticals, Inc | 010779978 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Lancaster Laboratories Inc | 069777290 | ANALYSIS | |