LORATADINE- loratadine tablet
Mylan Pharmaceuticals Inc.
----------
Drug Facts
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
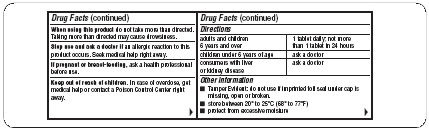
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Directions
|
adults and children |
1 tablet daily; not more |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver |
ask a doctor |
Other information
- •
- Tamper Evident: do not use if imprinted foil seal under cap is missing, open or broken.
- •
- store between 20° to 25°C (68° to 77°F)
- •
- protect from excessive moisture
Inactive ingredients
Anhydrous lactose, colloidal silicon dioxide, corn starch, hypromellose, magnesium stearate, microcrystalline cellulose, povidone and sodium lauryl sulfate.
PRODUCT PACKAGING
NDC 0378-1925-01
Original Prescription
Strength
Non-Drowsy*
Loratadine
Tablets USP, 10 mg
Antihistamine
Indoor and Outdoor Allergies
24 Hour Relief of:
- •
- Sneezing
- •
- Runny Nose
- •
- Itchy, Watery Eyes
- •
- Itchy Throat or Nose
*When taken as directed. See Drug Facts Panel.
100 TABLETS
RM1925A3
| LORATADINE
loratadine tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mylan Pharmaceuticals Inc. (059295980) |