GARDASIL
-
human papillomavirus type 6 l1 capsid protein antigen,
human papillomavirus type 11 l1 capsid protein antigen,
human papillomavirus type 16 l1 capsid protein antigen and
human papillomavirus type 18 l1 capsid protein antigen injection, suspension
Merck & Co., Inc.
----------
|
|||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Indications
GARDASIL®1 is a vaccine indicated in girls and women 9 through 26 years of age for the prevention of the following diseases caused by Human Papillomavirus (HPV) types included in the vaccine:
- Cervical, vulvar, and vaginal cancer caused by HPV types 16 and 18
- Genital warts (condyloma acuminata) caused by HPV types 6 and 11
And the following precancerous or dysplastic lesions caused by HPV types 6, 11, 16, and 18:
- Cervical intraepithelial neoplasia (CIN) grade 2/3 and Cervical adenocarcinoma in situ (AIS)
- Cervical intraepithelial neoplasia (CIN) grade 1
- Vulvar intraepithelial neoplasia (VIN) grade 2 and grade 3
- Vaginal intraepithelial neoplasia (VaIN) grade 2 and grade 3
1.2 Limitations of GARDASIL Use and Effectiveness
The health care provider should inform the patient, parent, or guardian that vaccination does not substitute for routine cervical cancer screening. Women who receive GARDASIL should continue to undergo cervical cancer screening per standard of care. [See Patient Counseling Information (17.1).]
GARDASIL has not been demonstrated to provide protection against disease from vaccine and non-vaccine HPV types to which a woman has previously been exposed through sexual activity. [See Clinical Studies (14.2).]
GARDASIL is not intended to be used for treatment of active genital warts; cervical, vulvar, and vaginal cancers; CIN; VIN; or VaIN.
GARDASIL has not been demonstrated to protect against diseases due to HPV types not contained in the vaccine. [See Clinical Studies (14.3)]
Not all vulvar and vaginal cancers are caused by HPV, and GARDASIL protects only against those vulvar and vaginal cancers caused by HPV 16 and 18.
GARDASIL does not protect against genital diseases not caused by HPV.
Vaccination with GARDASIL may not result in protection in all vaccine recipients.
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
GARDASIL should be administered intramuscularly as a 0.5-mL dose at the following schedule: 0, 2 months, 6 months. [See Clinical Studies (14.5).]
2.2 Method of Administration
Shake well before use. Thorough agitation immediately before administration is necessary to maintain suspension of the vaccine. GARDASIL should not be diluted or mixed with other vaccines. After thorough agitation, GARDASIL is a white, cloudy liquid. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use the product if particulates are present or if it appears discolored.
GARDASIL should be administered intramuscularly in the deltoid region of the upper arm or in the higher anterolateral area of the thigh.
Do not administer GARDASIL intravenously, intradermally, or subcutaneously.
Syncope has been reported following vaccination with GARDASIL and may result in falling with injury; observation for 15 minutes after administration is recommended. [See Warnings and Precautions (5.1)]
Single-Dose Vial Use
Withdraw the 0.5-mL dose of vaccine from the single-dose vial using a sterile needle and syringe and use promptly.
Prefilled Syringe Use With and Without Needle Guard (Safety) Device
Prefilled Syringe With Needle Guard (Safety) Device
Instructions for using the prefilled single-dose syringes preassembled with needle guard (safety) device
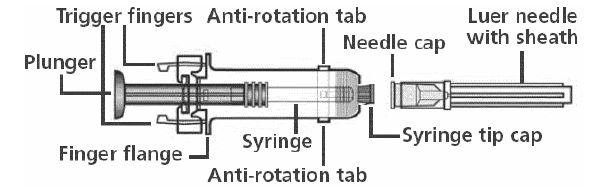
NOTE: Please use the enclosed needle for administration. If a different needle is chosen, it should fit securely on the syringe and be no longer than 1 inch to ensure proper functioning of the needle guard device. Two detachable labels are provided which can be removed after the needle is guarded.
At any of the following steps, avoid contact with the Trigger Fingers to keep from activating the safety device prematurely.
Remove Syringe Tip Cap and Needle Cap. Attach Luer Needle by pressing both Anti-Rotation Tabs to secure syringe and by twisting the Luer Needle in a clockwise direction until secured to the syringe. Remove Needle Sheath. Administer injection per standard protocol as stated above under DOSAGE AND ADMINISTRATION. Depress the Plunger while grasping the Finger Flange until the entire dose has been given. The Needle Guard Device will NOT activate to cover and protect the needle unless the ENTIRE dose has been given. While the Plunger is still depressed, remove needle from the vaccine recipient. Slowly release the Plunger and allow syringe to move up until the entire needle is guarded. For documentation of vaccination, remove detachable labels by pulling slowly on them. Dispose in approved sharps container.
Prefilled Syringe Without Needle Guard (Safety) Device
This package does not contain a needle guard (safety device) or a needle. Shake well before use. Attach the needle by twisting in a clockwise direction until the needle fits securely on the syringe. Administer the entire dose as per standard protocol.
3 DOSAGE FORMS AND STRENGTHS
GARDASIL is a suspension for intramuscular administration available in 0.5-mL single dose vials and prefilled syringes. See Description (11) for the complete listing of ingredients.
4 CONTRAINDICATIONS
Hypersensitivity, including severe allergic reactions to yeast (a vaccine component), or after a previous dose of GARDASIL. [See Description (11).]
5 WARNINGS AND PRECAUTIONS
5.1 Syncope
Because vaccinees may develop syncope, sometimes resulting in falling with injury, observation for 15 minutes after administration is recommended. Syncope, sometimes associated with tonic-clonic movements and other seizure-like activity, has been reported following vaccination with GARDASIL. When syncope is associated with tonic-clonic movements, the activity is usually transient and typically responds to restoring cerebral perfusion by maintaining a supine or Trendelenburg position.
5.2 Managing Allergic Reactions
Appropriate medical treatment and supervision must be readily available in case of anaphylactic reactions following the administration of GARDASIL.
6 ADVERSE REACTIONS
Overall Summary of Adverse Reactions
Headache, fever, nausea, and dizziness; and local injection site reactions (pain, swelling, erythema, pruritus, and bruising) occurred after administration with GARDASIL.
Syncope, sometimes associated with tonic-clonic movements and other seizure-like activity, has been reported following vaccination with GARDASIL and may result in falling with injury; observation for 15 minutes after administration is recommended. [See Warnings and Precautions (5.1)]
Anaphylaxis has been reported following vaccination with GARDASIL.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
Studies in Girls and Women 9 Through 26 Years of Age
In 5 clinical trials (3 Amorphous Aluminum Hydroxyphosphate Sulfate [AAHS]-controlled, 1 saline placebo-controlled, and 1 uncontrolled), 8878 subjects were administered GARDASIL or AAHS control or saline placebo on the day of enrollment, and approximately 2 and 6 months thereafter, and safety was evaluated using vaccination report cards (VRC)-aided surveillance for 14 days after each injection of GARDASIL or AAHS control or saline placebo in these subjects. The subjects who were monitored using VRC-aided surveillance included 5088 girls and women 9 through 26 years of age at enrollment who received GARDASIL and 3790 girls and women who received AAHS control or saline placebo. Few subjects (0.1%) discontinued due to adverse reactions. The race distribution of the study subjects was as follows: 62.3% White; 17.6% Hispanic (Black and White); 6.8% Asian; 6.7% Other; 6.4% Black; and 0.3% American Indian.
Common Injection Site Adverse Reactions in Girls and Women 9 Through 26 Years of Age
The injection site adverse reactions that were observed among recipients of GARDASIL at a frequency of at least 1.0% and also at a greater frequency than that observed among AAHS control or saline placebo recipients are shown in Table 1.
|
Adverse Reaction (1 to 5 Days Postvaccination) |
GARDASIL (N = 5088) % |
AAHS Control† (N = 3470) % |
Saline Placebo (N = 320) % |
|
Injection Site Pain Swelling Erythema Pruritus Bruising |
83.9 25.4 24.7 3.2 2.8 |
75.4 15.8 18.4 2.8 3.2 |
48.6 7.3 12.1 0.6 1.6 |
Evaluation of Injection-Site Adverse Reactions by Dose in Girls and Women 9 Through 26 Years of Age
An analysis of injection-site adverse reactions in girls and women by dose is shown in Table 2. Overall, 94.3% of girls and women who received GARDASIL judged their injection-site adverse reaction to be mild or moderate in intensity.
|
GARDASIL (% occurrence) |
AAHS Control* (% occurrence) |
Saline Placebo (% occurrence) |
|||||||
|
Adverse Reaction |
Post- dose 1 N† = 5011 |
Post- dose 2 N = 4924 |
Post- dose 3 N = 4818 |
Post- dose 1 N = 3410 |
Post- dose 2 N = 3351 |
Post- dose 3 N = 3295 |
Post- dose 1 N = 315 |
Post- dose 2 N = 301 |
Post- dose 3 N = 300 |
|
Pain Mild/Moderate Severe |
63.4 62.5 0.9 |
60.7 59.7 1.0 |
62.7 61.2 1.5 |
57.0 56.6 0.4 |
47.8 47.3 0.5 |
49.6 48.9 0.6 |
33.7 33.3 0.3 |
20.3 20.3 0.0 |
27.3 27.0 0.3 |
|
Swelling‡ Mild/Moderate Severe |
10.2 9.6 0.6 |
12.8 11.9 0.8 |
15.1 14.2 0.9 |
8.2 8.1 0.2 |
7.5 7.2 0.2 |
7.6 7.3 0.2 |
4.4 4.4 0.0 |
3.0 3.0 0.0 |
3.3 3.3 0.0 |
|
Erythema‡ Mild/Moderate Severe |
9.2 9.0 0.2 |
12.1 11.7 0.3 |
14.7 14.3 0.4 |
9.8 9.5 0.3 |
8.4 8.4 0.1 |
8.9 8.8 0.1 |
7.3 7.3 0.0 |
5.3 5.3 0.0 |
5.7 5.7 0.0 |
Common Systemic Adverse Reactions in Girls and Women 9 Through 26 Years of Age
Headache was the most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 28.2% and AAHS control or saline placebo = 28.4%). Fever was the next most commonly reported systemic adverse reaction in both treatment groups (GARDASIL = 13.0% and AAHS control or saline placebo = 11.2%).
Adverse reactions that were observed among recipients of GARDASIL, at a frequency of greater than or equal to 1.0% where the incidence in the GARDASIL group was greater than or equal to the incidence in the AAHS control or saline placebo group, are shown in Table 3.
|
Adverse Reactions (1 to 15 Days Postvaccination) |
GARDASIL (N = 5088) % |
AAHS control† or Saline Placebo (N = 3790) % |
| Pyrexia | 13.0 | 11.2 |
| Nausea | 6.7 | 6.5 |
| Dizziness | 4.0 | 3.7 |
| Diarrhea | 3.6 | 3.5 |
| Vomiting | 2.4 | 1.9 |
| Cough | 2.0 | 1.5 |
| Toothache | 1.5 | 1.4 |
| Upper respiratory tract infection | 1.5 | 1.5 |
| Malaise | 1.4 | 1.2 |
| Arthralgia | 1.2 | 0.9 |
| Insomnia | 1.2 | 0.9 |
| Nasal congestion | 1.1 | 0.9 |
Evaluation of Fever by Dose in Girls and Women 9 Through 26 Years of Age
An analysis of fever in girls and women by dose is shown in Table 4.
|
GARDASIL (% occurrence) |
AAHS Control* or Saline Placebo (% occurrence) |
|||||
|
Temperature (°F) |
Postdose 1 N† = 4945 |
Postdose 2 N = 4804 |
Postdose 3 N = 4671 |
Postdose 1 N = 3681 |
Postdose 2 N = 3564 |
Postdose 3 N = 3467 |
| ≥100 to <102 | 3.7 | 4.1 | 4.4 | 3.1 | 3.8 | 3.6 |
| ≥102 | 0.3 | 0.5 | 0.5 | 0.2 | 0.4 | 0.5 |
Serious Adverse Reactions in the Entire Study Population
A total of 237 subjects out of 25,274 total subjects (9- through 45-year-old girls and women; and 9- through 15-year-old boys) who received both GARDASIL (N = 13,686) and AAHS control (N = 11,004) or saline placebo (N = 584) reported a serious systemic adverse reaction following any vaccination visit during the clinical trials for GARDASIL.
Out of the entire study population (25,274 subjects), only 0.05% of the reported serious systemic adverse reactions were judged to be vaccine related by the study investigator. The most frequently reported serious systemic adverse reactions for GARDASIL compared to AAHS control or saline placebo and regardless of causality were:
Headache [0.02% GARDASIL (3 cases) vs. 0.02% AAHS Control (2 cases)],
Gastroenteritis [0.02% GARDASIL (3 cases) vs. 0.02% AAHS Control (2 cases)],
Appendicitis [0.03% GARDASIL (4 cases) vs. 0.01% AAHS Control (1 case)],
Pelvic inflammatory disease [0.02% GARDASIL (3 cases) vs. 0.04% AAHS Control (4 cases)],
Urinary tract infection [0.02% GARDASIL (2 cases) vs. 0.02% AAHS Control (2 cases)],
Pneumonia [0.02% GARDASIL (2 cases) vs. 0.02% AAHS Control (2 cases)],
Pyelonephritis [0.02% GARDASIL (2 cases) vs. 0.03% AAHS Control (3 cases)],
Pulmonary embolism [0.02% GARDASIL (2 cases) vs. 0.02% AAHS Control (2 cases)].
One case (0.007% GARDASIL: 0.0% AAHS Control or Saline Placebo) of bronchospasm; and 2 cases (0.02% GARDASIL: 0.0% AAHS Control or Saline Placebo) of asthma were reported as serious systemic adverse reactions that occurred following any vaccination visit.
In addition, there was 1 subject in the clinical trials, in the group that received GARDASIL, who reported two injection-site serious adverse reactions (injection-site pain and injection-site joint movement impairment).
Deaths in the Entire Study Population
Across the clinical studies, 24 deaths were reported in 25,274 (GARDASIL N = 13,686; AAHS Control N = 11,004, saline placebo N = 584) subjects (9- through 45-year-old girls and women; and 9- through 15-year-old boys). The events reported were consistent with events expected in healthy adolescent and adult populations. The most common cause of death was motor vehicle accident (4 subjects who received GARDASIL and 3 AAHS Control subjects), followed by overdose/suicide (2 subjects who received GARDASIL and 2 subjects who received AAHS Control), and pulmonary embolus/deep vein thrombosis (1 subject who received GARDASIL and 1 AAHS Control subject). In addition, there were 2 cases of sepsis, 1 case of pancreatic cancer, 1 case of arrhythmia, 1 case of pulmonary tuberculosis, 1 case of hyperthyroidism, 1 case of post-operative pulmonary embolism and acute renal failure, and 1 case of systemic lupus erythematosus in the group that received GARDASIL; 1 case of asphyxia, and 1 case of acute lymphocytic leukemia in the AAHS Control; and 1 case of medulloblastoma in the saline placebo group.
Systemic Autoimmune Disorders in Girls and Women 9 Through 26 Years of Age
In the clinical studies, 9- through 26-year-old girls and women were evaluated for new medical conditions that occurred over the course of follow-up. New medical conditions potentially indicative of a systemic autoimmune disorder seen in the group that received GARDASIL or AAHS control or saline placebo are shown in Table 5. This population includes all subjects who received at least one dose of GARDASIL or AAHS control or saline placebo, and had safety data available.
| Conditions |
GARDASIL (N = 10,706) |
AAHS Control* or Saline Placebo (N = 9412) |
| n (%) | n (%) | |
| N = Number of subjects enrolled n = Number of subjects with specific new Medical Conditions NOTE: Although a subject may have had two or more new Medical Conditions, the subject is counted only once within a category. The same subject may appear in different categories. |
||
|
||
| Arthralgia/Arthritis/Arthropathy† | 120 (1.1) | 98 (1.0) |
| Autoimmune Thyroiditis | 4 (0.0) | 1 (0.0) |
| Coeliac Disease | 10 (0.1) | 6 (0.1) |
| Diabetes Mellitus Insulin-dependent | 2 (0.0) | 2 (0.0) |
| Erythema Nodosum | 2 (0.0) | 4 (0.0) |
| Hyperthyroidism‡ | 27 (0.3) | 21 (0.2) |
| Hypothyroidism§ | 35 (0.3) | 38 (0.4) |
| Inflammatory Bowel Disease¶ | 7 (0.1) | 10 (0.1) |
| Multiple Sclerosis | 2 (0.0) | 4 (0.0) |
| Nephritis# | 2 (0.0) | 5 (0.1) |
| Optic Neuritis | 2 (0.0) | 0 (0.0) |
| Pigmentation DisorderÞ | 4 (0.0) | 3 (0.0) |
| Psoriasisß | 13 (0.1) | 15 (0.2) |
| Raynaud's Phenomenon | 3 (0.0) | 4 (0.0) |
| Rheumatoid Arthritisà | 6 (0.1) | 2 (0.0) |
| Scleroderma/Morphea | 2 (0.0) | 1 (0.0) |
| Stevens-Johnson Syndrome | 1 (0.0) | 0 (0.0) |
| Systemic Lupus Erythematosus | 1 (0.0) | 3 (0.0) |
| Uveitis | 3 (0.0) | 1 (0.0) |
| All Conditions | 245 (2.3) | 218 (2.3) |
Safety in Concomitant Use with RECOMBIVAX HB in Girls and Women 9 Through 26 Years of Age
The safety of GARDASIL when administered concomitantly with RECOMBIVAX HB hepatitis B vaccine (recombinant) was evaluated in an AAHS-controlled study of 1871 subjects with a mean age of 20.4 years. The race distribution of the study subjects was as follows: 61.6% White; 23.8% Other; 11.9% Black; 1.6% Hispanic (Black and White); 0.8% Asian; and 0.3% American Indian. The rates of systemic and injection-site adverse reactions were similar among subjects who received concomitant vaccination as compared with those who received GARDASIL or RECOMBIVAX HB hepatitis B vaccine.
6.2 Post-Marketing Experience
The following adverse events have been spontaneously reported during post-approval use of GARDASIL. Because these events were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or to establish a causal relationship to vaccine exposure.
Blood and lymphatic system disorders: Autoimmune hemolytic anemia, lymphadenopathy.
Respiratory, thoracic and mediastinal disorders: pulmonary embolus.
Gastrointestinal disorders: Nausea, pancreatitis, vomiting.
General disorders and administration site conditions: Asthenia, chills, death, fatigue, malaise.
Immune system disorders: Autoimmune diseases, hypersensitivity reactions including anaphylactic/anaphylactoid reactions, bronchospasm, and urticaria.
Musculoskeletal and connective tissue disorders: Arthralgia, myalgia.
Nervous system disorders: Dizziness, Guillain-Barré syndrome, headache, motor neuron disease, paralysis, seizures, syncope (including syncope associated with tonic-clonic movements and other seizure-like activity) sometimes resulting in falling with injury, transverse myelitis.
Vascular Disorders: Deep venous thrombosis.
7 DRUG INTERACTIONS
7.1 Use with RECOMBIVAX HB
Results from clinical studies indicate that GARDASIL may be administered concomitantly (at a separate injection site) with RECOMBIVAX HB hepatitis B vaccine (recombinant) [See Clinical Studies (14.6) ]. Co-administration of GARDASIL with other vaccines has not been studied.
7.2 Use with Hormonal Contraceptives
In clinical studies, 13,293 subjects (GARDASIL N = 6644; AAHS control or saline placebo N = 6649) who had post-Month 7 follow-up used hormonal contraceptives for a total of 17,597 person-years (65.1% of the total follow-up time in the studies). Use of hormonal contraceptives or lack of use of hormonal contraceptives among study participants did not alter immune response in the per protocol efficacy (PPE) population.
7.3 Use with Systemic Immunosuppressive Medications
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune responses to vaccines [see Use in Specific Populations (8.6)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B:
Reproduction studies have been performed in female rats at doses up to 300 times the human dose (on a mg/kg basis) and have revealed no evidence of impaired female fertility or harm to the fetus due to GARDASIL. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human responses, GARDASIL should be used during pregnancy only if clearly needed.
An evaluation of the effect of GARDASIL on embryo-fetal, pre- and postweaning development was conducted using rats. One group of rats was administered GARDASIL twice prior to gestation, during the period of organogenesis (gestation Day 6) and on lactation Day 7. A second group of pregnant rats was administered GARDASIL during the period of organogenesis (gestation Day 6) and on lactation Day 7 only. GARDASIL was administered at 0.5 mL/rat/occasion (approximately 300-fold excess relative to the projected human dose on a mg/kg basis) by intramuscular injection. No adverse effects on mating, fertility, pregnancy, parturition, lactation, embryo-fetal or pre- and postweaning development were observed. There were no vaccine-related fetal malformations or other evidence of teratogenesis noted in this study. In addition, there were no treatment-related effects on developmental signs, behavior, reproductive performance, or fertility of the offspring. The effect of GARDASIL on male fertility has not been studied.
In clinical studies, women underwent urine pregnancy testing prior to administration of each dose of GARDASIL. Women who were found to be pregnant before completion of a 3-dose regimen of GARDASIL were instructed to defer completion of their vaccination regimen until resolution of the pregnancy.
GARDASIL is not indicated for women 27 years of age or older. However, safety data in women 16 through 45 years of age was collected, and 3620 women (GARDASIL N = 1796 vs. AAHS control or saline placebo N = 1824) reported at least 1 pregnancy each.
The overall proportions of pregnancies that resulted in an adverse outcome, defined as the combined numbers of spontaneous abortion, late fetal death, and congenital anomaly cases out of the total number of pregnancy outcomes for which an outcome was known (and excluding elective terminations), were 23.3% (423/1812) in subjects who received GARDASIL and 24.1% (438/1820) in subjects who received AAHS control or saline placebo.
Overall, 54 and 63 subjects in the group that received GARDASIL or AAHS control or saline placebo, respectively (3.0% and 3.5% of all subjects who reported a pregnancy in the respective vaccination groups), experienced a serious adverse reaction during pregnancy. The most common events reported were conditions that can result in Caesarean section (e.g., failure of labor, malpresentation, cephalopelvic disproportion), premature onset of labor (e.g., threatened abortions, premature rupture of membranes), and pregnancy-related medical problems (e.g., pre-eclampsia, hyperemesis). The proportions of pregnant subjects who experienced such events were comparable between the groups receiving GARDASIL and AAHS control or saline placebo.
There were 40 cases of congenital anomaly in pregnancies that occurred in subjects who received GARDASIL and 30 cases of congenital anomaly in pregnancies that occurred in subjects who received AAHS control or saline placebo.
Further sub-analyses were conducted to evaluate pregnancies with estimated onset within 30 days or more than 30 days from administration of a dose of GARDASIL or AAHS control or saline placebo. For pregnancies with estimated onset within 30 days of vaccination, 5 cases of congenital anomaly were observed in the group that received GARDASIL compared to 1 case of congenital anomaly in the group that received AAHS control or saline placebo. The congenital anomalies seen in pregnancies with estimated onset within 30 days of vaccination included pyloric stenosis, congenital megacolon, congenital hydronephrosis, hip dysplasia, and club foot. Conversely, in pregnancies with onset more than 30 days following vaccination, 35 cases of congenital anomaly were observed in the group that received GARDASIL compared with 29 cases of congenital anomaly in the group that received AAHS control or saline placebo.
Pregnancy Registry for GARDASIL
Merck & Co., Inc. maintains a Pregnancy Registry to monitor fetal outcomes of pregnant women exposed to GARDASIL. Patients and health care providers are encouraged to report any exposure to GARDASIL during pregnancy by calling (800) 986-8999.
8.3 Nursing Mothers
Women 16 Through 26 Years of Age
It is not known whether GARDASIL is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when GARDASIL is administered to a nursing woman.
A total of 995 nursing mothers (vaccine N = 500, AAHS control N = 495) were given GARDASIL or AAHS control during the vaccination period of the clinical trials.
Overall, 21 and 10 infants of subjects who received GARDASIL or AAHS control, respectively (representing 4.2% and 2.0% of the total number of subjects who were breast-feeding during the period in which they received GARDASIL or AAHS control, respectively), experienced a serious adverse reaction.
In a post-hoc analysis of clinical studies, a higher number of breast-feeding infants (n = 6) whose mothers received GARDASIL had acute respiratory illnesses within 30 days post-vaccination of the mother as compared to infants (n = 2) whose mothers received AAHS control. In these studies, the rates of other adverse reactions in the mother and the nursing infant were comparable between vaccination groups.
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients below 9 years of age nor in pediatric males of any age.
Clinical studies of pediatric males 9 through 15 years of age were conducted and contributed safety data to the serious adverse reactions and deaths. [See Adverse Reactions (6.1).]
8.5 Geriatric Use
The safety and effectiveness of GARDASIL have not been evaluated in subjects aged 65 years and over.
8.6 Immunocompromised Individuals
The immunologic response to GARDASIL may be diminished in immunocompromised individuals [see Drug Interactions (7.3)].
10 OVERDOSAGE
There have been reports of administration of higher than recommended doses of GARDASIL.
In general, the adverse event profile reported with overdose was comparable to recommended single doses of GARDASIL.
11 DESCRIPTION
GARDASIL, Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant, is a non-infectious recombinant quadrivalent vaccine prepared from the purified virus-like particles (VLPs) of the major capsid (L1) protein of HPV Types 6, 11, 16, and 18. The L1 proteins are produced by separate fermentations in recombinant Saccharomyces cerevisiae and self-assembled into VLPs. The fermentation process involves growth of S. cerevisiae on chemically-defined fermentation media which include vitamins, amino acids, mineral salts, and carbohydrates. The VLPs are released from the yeast cells by cell disruption and purified by a series of chemical and physical methods. The purified VLPs are adsorbed on preformed aluminum-containing adjuvant (Amorphous Aluminum Hydroxyphosphate Sulfate). The quadrivalent HPV VLP vaccine is a sterile liquid suspension that is prepared by combining the adsorbed VLPs of each HPV type and additional amounts of the aluminum-containing adjuvant and the final purification buffer.
GARDASIL is a sterile suspension for intramuscular administration. Each 0.5-mL dose contains approximately 20 mcg of HPV 6 L1 protein, 40 mcg of HPV 11 L1 protein, 40 mcg of HPV 16 L1 protein, and 20 mcg of HPV 18 L1 protein.
Each 0.5-mL dose of the vaccine contains approximately 225 mcg of aluminum (as Amorphous Aluminum Hydroxyphosphate Sulfate adjuvant), 9.56 mg of sodium chloride, 0.78 mg of L-histidine, 50 mcg of polysorbate 80, 35 mcg of sodium borate, < 7 mcg yeast protein/dose, and water for injection. The product does not contain a preservative or antibiotics.
After thorough agitation, GARDASIL is a white, cloudy liquid.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
HPV only infects human beings. Animal studies with analogous animal papillomaviruses suggest that the efficacy of L1 VLP vaccines may involve the development of humoral immune responses. Human beings develop a humoral immune response to the vaccine, although the exact mechanism of protection is unknown.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
GARDASIL has not been evaluated for the potential to cause carcinogenicity or genotoxicity.
GARDASIL administered to female rats at a dose of 120 mcg total protein, which corresponds to approximately 300-fold excess relative to the projected human dose, had no effects on mating performance, fertility, or embryonic/fetal survival.
14 CLINICAL STUDIES
CIN 2/3 and AIS are the immediate and necessary precursors of squamous cell carcinoma and adenocarcinoma of the cervix, respectively. Their detection and removal has been shown to prevent cancer; thus, they serve as surrogate markers for prevention of cervical cancer. In the clinical studies, cases of CIN 2/3 and AIS were the efficacy endpoints to assess prevention of cervical cancer. In addition, cases of vulvar and vaginal intraepithelial neoplasia (VIN 2/3 and VaIN 2/3) were the efficacy endpoints to assess prevention of HPV-related vulvar and vaginal cancers, and observations of external genital lesions were the efficacy endpoints for the prevention of genital warts.
Efficacy was assessed in 4 AAHS-controlled, double-blind, randomized Phase II and III clinical studies. The first Phase II study evaluated the HPV 16 component of GARDASIL (Protocol 005 or Study 1, N = 2391) and the second evaluated all components of GARDASIL (Protocol 007 or Study 2, N = 551). The Phase III studies evaluated GARDASIL in 5442 (Study 3 or Protocol 013 [FUTURE I]) and 12,157 (Study 4 or Protocol 015 [FUTURE II]) subjects. Together, these four studies evaluated 20,541 women 16 through 26 years of age at enrollment with a mean age of 20.0 years. The race distribution of the study subjects was as follows: 70.4% White; 12.2% Hispanic (Black and White); 8.8% Other; 4.6% Black; 3.8% Asian; and 0.2% American Indian. The median duration of follow-up was 4.0, 3.0, 3.0, and 3.0 years for Study 1, Study 2, Study 3, and Study 4, respectively. Subjects received vaccine or AAHS control on the day of enrollment and 2 and 6 months thereafter. Efficacy was analyzed for each study individually and for all studies combined according to a prospective clinical plan.
Overall, 73% of subjects were naïve (i.e., PCR [Polymerase Chain Reaction] negative and seronegative for all 4 vaccine HPV types) to all 4 vaccine HPV types at enrollment.
A total of 27% of subjects had evidence of prior exposure to or ongoing infection with at least 1 of the 4 vaccine HPV types. Among these subjects, 74% had evidence of prior exposure to or ongoing infection with only 1 of the 4 vaccine HPV types and were naïve (PCR negative and seronegative) to the remaining 3 types.
In subjects who were naïve (PCR negative and seronegative) to all 4 vaccine HPV types, CIN, genital warts, VIN, and VaIN caused by any of the 4 vaccine HPV types were counted as endpoints.
Among subjects who were positive (PCR positive and/or seropositive) for a vaccine HPV type at Day 1, endpoints related to that type were not included in the analyses of prophylactic efficacy. Endpoints related to the remaining types for which the subject was naïve (PCR negative and seronegative) were counted.
For example, in subjects who were HPV 18 positive (PCR positive and/or seropositive) at Day 1, lesions caused by HPV 18 were not counted in the prophylactic efficacy evaluations. Lesions caused by HPV 6, 11, and 16 were included in the prophylactic efficacy evaluations. The same approach was used for the other types.
14.1 Prophylactic Efficacy – HPV Types 6, 11, 16, and 18 in Women 16 Through 26 Years of Age
GARDASIL was administered without prescreening for presence of HPV infection and the efficacy trials allowed enrollment of subjects regardless of baseline HPV status (i.e., PCR status or serostatus). Subjects with current or prior HPV infection with an HPV type contained in the vaccine were not eligible for prophylactic efficacy evaluations for that type.
The primary analyses of efficacy with respect to HPV types 6, 11, 16, and 18 were conducted in the per-protocol efficacy (PPE) population, consisting of individuals who received all 3 vaccinations within 1 year of enrollment, did not have major deviations from the study protocol, and were naïve (PCR negative in cervicovaginal specimens and seronegative) to the relevant HPV type(s) (Types 6, 11, 16, and 18) prior to dose 1 and through 1 month Postdose 3 (Month 7). Efficacy was measured starting after the Month 7 visit.
GARDASIL was efficacious in reducing the incidence of CIN (any grade including CIN 2/3); AIS; genital warts; VIN (any grade); and VaIN (any grade) related to vaccine HPV types 6, 11, 16, or 18 in those who were PCR negative and seronegative at baseline (Table 6).
In addition, individuals who were already infected with 1 or more vaccine-related HPV types prior to vaccination were protected from precancerous cervical lesions and external genital lesions caused by the other vaccine HPV types.
| Population | GARDASIL | AAHS Control | % Efficacy (95% CI) | ||
| N | Number of cases | N | Number of cases | ||
| N = Number of subjects with at least 1 follow-up visit after Month 7 CI = Confidence Interval Note 1: Point estimates and confidence intervals are adjusted for person-time of follow-up Note 2: The first analysis in the table (i.e., HPV 16- or 18-related CIN 2/3, AIS or worse) was the primary endpoint of the vaccine development plan. Note 3: Study 1 = Protocol 005; Study 2 = Protocol 007; Study 3 = Protocol 013; and Study 4 = Protocol 015 Note 4: Table 6 does not include cases due to non-vaccine HPV types AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate |
|||||
|
|||||
| HPV 16- or 18-related CIN 2/3 or AIS | |||||
| Study 1‡ | 755 | 0 | 750 | 12 | 100.0 (65.1, 100.0) |
| Study 2 | 231 | 0 | 230 | 1 | 100.0 (-3744.9, 100.0) |
| Study 3 | 2201 | 0 | 2222 | 36 | 100.0 (89.2, 100.0) |
| Study 4 | 5306 | 2 | 5262 | 63 | 96.9 (88.2, 99.6) |
| Combined Protocols§ | 8493 | 2 | 8464 | 112 | 98.2 (93.5, 99.8) |
| HPV 16-related CIN 2/3 or AIS | |||||
| Combined Protocols§ | 7402 | 2 | 7205 | 93 | 97.9 (92.3, 99.8) |
| HPV 18-related CIN 2/3 or AIS | |||||
| Combined Protocols§ | 7382 | 0 | 7316 | 29 | 100.0 (86.6, 100.0) |
| HPV 16- or 18-related VIN 2/3 | |||||
| Study 2 | 231 | 0 | 230 | 0 | Not calculated |
| Study 3 | 2219 | 0 | 2239 | 6 | 100.0 (14.4, 100.0) |
| Study 4 | 5322 | 0 | 5275 | 4 | 100.0 (-50.3, 100.0) |
| Combined Protocols§ | 7772 | 0 | 7744 | 10 | 100.0 (55.5, 100.0) |
| HPV 16- or 18-related VaIN 2/3 | |||||
| Study 2 | 231 | 0 | 230 | 0 | Not calculated |
| Study 3 | 2219 | 0 | 2239 | 5 | 100.0 (-10.1, 100.0) |
| Study 4 | 5322 | 0 | 5275 | 4 | 100.0 (-50.3, 100.0) |
| Combined Protocols§ | 7772 | 0 | 7744 | 9 | 100.0 (49.5, 100.0) |
| HPV 6-, 11-, 16-, or 18-related CIN (CIN 1, CIN 2/3) or AIS | |||||
| Study 2 | 235 | 0 | 233 | 3 | 100.0 (-138.4, 100.0) |
| Study 3 | 2241 | 0 | 2258 | 77 | 100.0 (95.1, 100.0) |
| Study 4 | 5388 | 9 | 5374 | 145 | 93.8 (88.0, 97.2) |
| Combined Protocols§ | 7864 | 9 | 7865 | 225 | 96.0 (92.3, 98.2) |
| HPV 6-, 11-, 16-, or 18-related Genital Warts | |||||
| Study 2 | 235 | 0 | 233 | 3 | 100.0 (-139.5, 100.0) |
| Study 3 | 2261 | 0 | 2279 | 58 | 100.0 (93.5, 100.0) |
| Study 4 | 5404 | 2 | 5390 | 132 | 98.5 (94.5, 99.8) |
| Combined Protocols§ | 7900 | 2 | 7902 | 193 | 99.0 (96.2, 99.9) |
| HPV 6- and 11-related Genital Warts | |||||
| Combined Protocols§ | 6932 | 2 | 6856 | 189 | 99.0 (96.2, 99.9) |
Prophylactic efficacy against overall cervical and genital disease related to HPV 6, 11, 16, and 18 in an extension phase of Study 2, that included data through month 60, was noted to be 100% (95% CI: 12.3%, 100.0%) among subjects in the per protocol population naïve to the relevant HPV types.
GARDASIL was efficacious against HPV disease caused by HPV types 6, 11, 16, and 18 in women who were naïve for those specific HPV types at baseline.
14.2 Effectiveness of GARDASIL in Prevention of HPV Types 6-, 11-, 16-, or 18-Related Disease in Women 16 Through 26 Years of Age, Regardless of Current or Prior Exposure to Vaccine HPV Types
The clinical trials included women regardless of current or prior exposure to vaccine HPV types, and additional analyses were conducted to evaluate the impact of GARDASIL with respect to HPV 6-, 11-, 16-, and 18-related cervical and genital disease in these women. Here, analyses included events arising among women regardless of baseline PCR status and serostatus, including HPV infections that were present at the start of vaccination as well as events that arose from infections that were acquired after the start of vaccination.
The impact of GARDASIL in women regardless of current or prior exposure to a vaccine HPV type is shown in Table 7. Impact was measured starting 1 month Postdose 1. Prophylactic efficacy denotes the vaccine’s efficacy in women who are naïve (PCR negative and seronegative) to the relevant HPV types at vaccination onset. Vaccine impact in women who were positive for vaccine HPV infection, as well as vaccine impact among women regardless of baseline vaccine HPV PCR status and serostatus are also presented. The majority of CIN and genital warts, VIN, and VaIN related to a vaccine HPV type detected in the group that received GARDASIL occurred as a consequence of HPV infection with the relevant HPV type that was already present at Day 1.
There was no clear evidence of protection from disease caused by HPV types for which women were PCR positive regardless of serostatus at baseline.
| Endpoints | Analysis | GARDASIL or HPV 16 L1 VLP Vaccine | AAHS Control | % Reduction (95% CI) |
||
| N | Cases | N | Cases | |||
| CI = Confidence Interval N = Number of subjects who have at least one follow-up visit after Day 1 Note 1: The 16- and 18-related CIN 2/3 or AIS composite endpoint included data from studies 1, 2, 3, and 4. All other endpoints only included data from studies 2, 3, and 4. Note 2: Positive status at Day 1 denotes PCR positive and/or seropositive for the respective type at Day 1 Note 3: Table 7 does not include disease due to non-vaccine HPV types AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate |
||||||
|
||||||
| HPV 16- or 18-related CIN 2/3 or AIS | Prophylactic Efficacy* | 9346 | 4 | 9407 | 155 | 97.4 (93.3, 99.3) |
| HPV 16 and/or HPV 18 Positive at Day 1 | 2870 | 142 | 2898 | 148† | -- | |
| Women regardless of Current or Prior Exposure to HPV 16 or 18‡ | 9836 | 146 | 9904 | 303 | 51.8 (41.1, 60.7)§ | |
| HPV 16- or 18-related VIN 2/3 or VaIN 2/3 | Prophylactic Efficacy* | 8642 | 1 | 8673 | 34 | 97.0 (82.4, 99.9) |
| HPV 16 and/or HPV 18 Positive at Day 1 | 1880 | 8 | 1876 | 4 | -- | |
| Women regardless of Current or Prior Exposure to HPV 16 or 18‡ | 8955 | 9 | 8968 | 38 | 76.3 (50.0, 89.9)§ | |
| HPV 6-, 11-, 16-, 18-related CIN (CIN 1, CIN 2/3) or AIS | Prophylactic Efficacy* | 8630 | 16 | 8680 | 309 | 94.8 (91.5, 97.1) |
| HPV 6, HPV 11, HPV 16, and/or HPV 18 Positive at Day 1 | 2466 | 186¶ | 2437 | 213¶ | -- | |
| Women regardless of Current or Prior Exposure to Vaccine HPV Types‡ | 8819 | 202 | 8854 | 522 | 61.5 (54.6, 67.4)§ | |
| HPV 6-, 11-, 16-, or 18-related Genital Warts | Prophylactic Efficacy* | 8761 | 10 | 8792 | 252 | 96.0 (92.6, 98.1) |
| HPV 6, HPV 11, HPV 16, and/or HPV 18 Positive at Day 1 | 2501 | 51# | 2475 | 55# | -- | |
| Women regardless of Current or Prior Exposure to Vaccine HPV Types‡ | 8955 | 61 | 8968 | 307 | 80.3 (73.9, 85.3)§ | |
| HPV 6- or 11-related Genital Warts | Prophylactic Efficacy* | 7769 | 9 | 7792 | 246 | 96.4 (93.0, 98.4) |
| HPV 6 and/or HPV 11 Positive at Day 1 | 1186 | 51 | 1176 | 54 | -- | |
| Women regardless of Current or Prior Exposure to Vaccine HPV Types‡ | 8955 | 60 | 8968 | 300 | 80.1 (73.7, 85.2)§ | |
14.3 Effectiveness of GARDASIL in Prevention of Any HPV Type Related Genital Disease in Women 16 Through 26 Years of Age, Regardless of Current or Prior Infection with Vaccine or Non-Vaccine HPV Types
The impact of GARDASIL against the overall burden of HPV-related cervical, vulvar, and vaginal disease (i.e., disease caused by any HPV type) results from a combination of prophylactic efficacy against vaccine HPV types, disease contribution from vaccine HPV types present at time of vaccination, and the disease contribution from HPV types not contained in the vaccine.
Additional efficacy analyses were conducted in 2 populations: (1) a generally HPV-naïve population (negative to 14 common HPV types and had a Pap test that was negative for SIL [Squamous Intraepithelial Lesion] at Day 1), approximating a population of sexually-naïve adolescents and young adult women and (2) the general study population of young adult women regardless of baseline HPV status, some of whom had HPV-related disease at vaccination onset.
Among generally HPV-naïve women and among all women in the study population (including women with HPV infection at vaccination onset), GARDASIL reduced the overall incidence of CIN 2/3 or AIS; of VIN 2/3 or VaIN 2/3; of CIN (any grade) or AIS; and of Genital Warts (Table 8). These reductions were primarily due to reductions in lesions caused by HPV types 6, 11, 16, and 18 in women naïve (seronegative and PCR negative) for the specific relevant vaccine HPV type. Infected women may already have CIN 2/3 or AIS at vaccination onset and some will develop CIN 2/3 or AIS during follow-up, either related to a vaccine or non-vaccine HPV type present at the time of vaccination or related to a non-vaccine HPV type not present at the time of vaccination.
| Endpoints Caused by Vaccine or Non-vaccine HPV Types | Analysis | GARDASIL | AAHS Control | % Reduction (95% CI) |
||
| N | Cases | N | Cases | |||
| CI = Confidence Interval AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate |
||||||
|
||||||
| CIN 2/3 or AIS | Prophylactic Efficacy* | 4616 | 77 | 4680 | 136 | 42.7 (23.7, 57.3) |
| Women regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† | 8559 | 421 | 8592 | 516 | 18.4 (7.0, 28.4)‡ | |
| VIN 2/3 and VaIN 2/3 | Prophylactic Efficacy* | 4688 | 7 | 4735 | 31 | 77.1 (47.1, 91.5) |
| Women regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† | 8688 | 30 | 8701 | 61 | 50.7 (22.5, 69.3)‡ | |
| CIN (Any Grade) or AIS | Prophylactic Efficacy* | 4616 | 272 | 4680 | 390 | 29.7 (17.7, 40.0) |
| Women regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† | 8559 | 967 | 8592 | 1189 | 19.1 (11.9, 25.8)‡ | |
| Genital Warts | Prophylactic Efficacy* | 4688 | 29 | 4735 | 169 | 82.8 (74.3, 88.8) |
| Women regardless of Current or Prior Exposure to Vaccine or Non-Vaccine HPV Types† | 8688 | 132 | 8701 | 350 | 62.5 (54.0, 69.5)‡ | |
The overall efficacy of GARDASIL will vary with the baseline prevalence of HPV infection and disease, the incidence of infections against which GARDASIL has shown protection, and those infections against which GARDASIL has not been shown to protect.
The efficacy of GARDASIL for HPV types not included in the vaccine (i.e., cross-protective efficacy) is a component of the overall impact of the vaccine on rates of CIN 2/3 or worse caused by HPV. Cross-protective efficacy was not demonstrated against disease caused by non-vaccine HPV types in the combined database of the Study 3 and Study 4 trials.
GARDASIL does not protect against genital disease not related to HPV. One subject who received GARDASIL in Study 3 developed an external genital well-differentiated squamous cell carcinoma at month 24. No HPV DNA was detected in the lesion or in any other samples taken throughout the study.
In 18,150 subjects enrolled in Study 2, Study 3, and Study 4, GARDASIL reduced definitive cervical therapy procedures by 23.9% (95% CI: 15.2%, 31.7%).
14.4 Other Studies
Data are insufficient to establish effectiveness of GARDASIL in women 27 through 45 years of age.
14.5 Immunogenicity
Assays to Measure Immune Response
The minimum anti-HPV titer that confers protective efficacy has not been determined.
Because there were few disease cases in subjects naïve (PCR negative and seronegative) to vaccine HPV types at baseline in the group that received GARDASIL, it has not been possible to establish minimum anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 antibody levels that protect against clinical disease caused by HPV 6, 11, 16, and/or 18.
The immunogenicity of GARDASIL was assessed in 20,132 9- through 26-year-old girls and women (GARDASIL N = 10,723; AAHS control or saline placebo N = 9409).
Type-specific immunoassays with type-specific standards were used to assess immunogenicity to each vaccine HPV type. These assays measured antibodies against neutralizing epitopes for each HPV type. The scales for these assays are unique to each HPV type; thus, comparisons across types and to other assays are not appropriate.
Immune Response to GARDASIL
The primary immunogenicity analyses were conducted in a per-protocol immunogenicity (PPI) population. This population consisted of individuals who were seronegative and PCR negative to the relevant HPV type(s) at enrollment, remained HPV PCR negative to the relevant HPV type(s) through 1 month postdose 3 (month 7), received all 3 vaccinations, and did not deviate from the study protocol in ways that could interfere with the effects of the vaccine.
Immunogenicity was measured by (1) the percentage of subjects who were seropositive for antibodies against the relevant vaccine HPV type, and (2) the Geometric Mean Titer (GMT).
In clinical studies, 99.8%, 99.8%, 99.8%, and 99.5% of girls and women who received GARDASIL became anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 seropositive, respectively, by 1 month postdose 3 across all age groups tested. Anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 GMTs peaked at month 7. GMTs declined through month 24 and then stabilized through month 36 at levels above baseline (Table 9). The duration of immunity following a complete schedule of immunization with GARDASIL has not been established.
| Study Time |
GARDASIL N† = 276 |
AAHS Control N = 275 |
||
| n‡ |
Geometric Mean Titer (95% CI) mMU/mL§ | n |
Geometric Mean Titer (95% CI) mMU/mL |
|
| Note: These data are from Study 2. AAHS Control = Amorphous Aluminum Hydroxyphosphate Sulfate CI=Confidence Interval |
||||
|
||||
| Anti-HPV 6 | ||||
| Month 07 | 208 | 582.2 (527.2, 642.8) | 198 | 4.6 (4.3, 4.8) |
| Month 24 | 192 | 93.7 (82.2, 106.9) | 188 | 4.6 (4.3, 5.0) |
| Month 36 | 183 | 93.8 (81.0, 108.6) | 184 | 5.1 (4.7, 5.6) |
| Anti-HPV 11 | ||||
| Month 07 | 208 | 696.5 (617.8, 785.2) | 198 | 4.1 (4.0, 4.2) |
| Month 24 | 190 | 97.1 (84.2, 112.0) | 188 | 4.2 (4.0, 4.3) |
| Month 36 | 174 | 91.7 (78.3, 107.3) | 180 | 4.4 (4.1, 4.7) |
| Anti-HPV 16 | ||||
| Month 07 | 193 | 3889.0 (3318.7, 4557.4) | 185 | 6.5 (6.2, 6.9) |
| Month 24 | 174 | 393.0 (335.7, 460.1) | 175 | 6.8 (6.3, 7.4) |
| Month 36 | 176 | 507.3 (434.6, 592.0) | 170 | 7.7 (6.8, 8.8) |
| Anti-HPV 18 | ||||
| Month 07 | 219 | 801.2 (693.8, 925.4) | 209 | 4.6 (4.3, 5.0) |
| Month 24 | 204 | 59.9 (49.7, 72.2) | 199 | 4.6 (4.3, 5.0) |
| Month 36 | 196 | 59.7 (48.5, 73.5) | 193 | 4.8 (4.4, 5.2) |
Bridging the Efficacy of GARDASIL from Young Adult Women to Adolescent Girls
A clinical study compared anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 GMTs in 10- through 15-year-old girls with responses in 16- through 23-year-old young women. Among subjects who received GARDASIL, 99.1% to 100% became anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 seropositive by 1 month postdose 3.
Table 10 compares the 1 month postdose 3 anti-HPV 6, anti-HPV 11, anti-HPV 16, and anti-HPV 18 GMTs in 9- through 15-year-old girls with those in 16- through 26-year-old young women.
|
Assay (cLIA) |
9- Through 15-year-old Female Adolescents (Protocols 016 and 018) N* = 1121 |
16- Through 26-year-old Adult Women (Protocols 013 and 015) N = 4229 |
||||
| n† | GMT‡ | (95% CI) | n | GMT | (95% CI) | |
| CI = Confidence Interval | ||||||
| Anti-HPV 6 | 915 | 928.7 | (874.0, 986.8) | 2631 | 542.6 | (526.2, 559.6) |
| Anti-HPV 11 | 915 | 1303.0 | (1223.1, 1388.0) | 2655 | 761.5 | (735.3, 788.6) |
| Anti-HPV 16 | 913 | 4909.2 | (4547.6, 5299.5) | 2570 | 2293.9 | (2185.0, 2408.2) |
| Anti-HPV 18 | 920 | 1039.8 | (964.9, 1120.4) | 2796 | 461.6 | (444.0, 480.0) |
Anti-HPV responses 1 month postdose 3 among 9- through 15-year-old girls were non-inferior to anti-HPV responses in 16- through 26-year-old young women in the combined database of immunogenicity studies for GARDASIL.
On the basis of this immunogenicity bridging, the efficacy of GARDASIL in 9- through 15-year-old girls is inferred.
GMT Response to Variation in Dosing Regimen in 18- Through 26-Year-Old Women
Subjects evaluated in the PPE population of clinical studies received all 3 vaccinations within 1 year of enrollment. An analysis of immune response data suggests that flexibility of ±1 month for Dose 2 (i.e., Month 1 to Month 3 in the vaccination regimen) and flexibility of ±2 months for Dose 3 (i.e., Month 4 to Month 8 in the vaccination regimen) do not impact the immune responses to GARDASIL.
Duration of the Immune Response to GARDASIL
The duration of immunity following a complete schedule of immunization with GARDASIL has not been established. The peak anti-GMTs for HPV types 6, 11, 16, and 18 occurred at month 7. Anti-GMTs for HPV types 6, 11, 16, and 18 were similar between measurements at month 24 and month 60 in Study 2.
14.6 Studies with RECOMBIVAX HB
The safety and immunogenicity of co-administration of GARDASIL with RECOMBIVAX HB hepatitis B vaccine (recombinant) (same visit, injections at separate sites) were evaluated in a randomized study of 1871 women aged 16 through 24 years at enrollment. Immune response to both hepatitis B vaccine (recombinant) and GARDASIL was non-inferior whether they were administered at the same visit or at a different visit.
16 HOW SUPPLIED/STORAGE AND HANDLING
All presentations for GARDASIL contain a suspension of 120 mcg L1 protein from HPV types 6, 11, 16, and 18 in a 0.5 mL dose. GARDASIL is supplied in vials and syringes.
Carton of one 0.5-mL single-dose vial, NDC 0006-4045-00.
Carton of ten 0.5-mL single-dose vials, NDC 0006-4045-41.
Carton of six 0.5-mL single-dose prefilled Luer Lock syringes, preassembled with UltraSafe Passive®2 delivery system. One-inch, 25-gauge needles are provided separately in the package. NDC 0006-4109-06.
Carton of six 0.5-mL single-dose prefilled Luer Lock syringes with tip caps. NDC 0006-4109-09.
Store refrigerated at 2 to 8°C (36 to 46°F). Do not freeze. Protect from light.
GARDASIL should be administered as soon as possible after being removed from refrigeration.
GARDASIL can be out of refrigeration (at temperatures at or below 25°C/77°F), for a total time of not more than 72 hours.
17 PATIENT COUNSELING INFORMATION
[See FDA-Approved Patient Labeling (17.2).]
17.1 Information for the Patient, Parent, or Guardian
Inform the patient, parent, or guardian:
- Vaccination does not substitute for routine cervical cancer screening. Women who receive GARDASIL should continue to undergo cervical cancer screening per standard of care.
- GARDASIL has not been demonstrated to provide protection against disease from vaccine and non-vaccine HPV types to which a woman has previously been exposed through sexual activity.
- Since syncope has been reported following vaccination sometimes resulting in falling with injury, observation for 15 minutes after administration is recommended.
- Vaccine information is required to be given with each vaccination to the patient, parent, or guardian.
- Information regarding benefits and risks associated with vaccination.
- GARDASIL is not recommended for use in pregnant women.
- Importance of completing the immunization series unless contraindicated.
- Report any adverse reactions to their health care provider.
Manufactured and Distributed by:
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Printed in USA
9883611
1Registered trademark of MERCK & CO., Inc. Whitehouse Station, NJ 08889, USA
COPYRIGHT © 2006, 2008 MERCK & CO., Inc.
All rights reserved
2UltraSafe Passive® delivery system is a Trademark of Safety Syringes, Inc.
17.2 FDA-Approved Patient Labeling
USPPI
Patient Information about
GARDASIL® (pronounced "gard-Ah-sill")
Generic name: [Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant]
Read this information with care before getting GARDASIL1. You (the person getting GARDASIL) will need 3 doses of the vaccine. It is important to read this leaflet when you get each dose. This leaflet does not take the place of talking with your health care provider about GARDASIL.
What is GARDASIL?
GARDASIL is a vaccine (injection/shot) that is used for girls and women 9 through 26 years of age to help protect against the following diseases caused by Human Papillomavirus (HPV):
- Cervical cancer
- Vulvar and vaginal cancers
- Genital warts
- Abnormal and precancerous cervical, vaginal, and vulvar lesions
- The diseases listed above have many causes, and GARDASIL only protects against diseases caused by certain kinds of HPV (called Type 6, Type 11, Type 16, and Type 18). Most of the time, these 4 types of HPV are responsible for the diseases listed above.
- GARDASIL cannot protect you from a disease that is caused by other types of HPV, other viruses, or bacteria.
- GARDASIL does not treat HPV infection.
- You cannot get HPV or any of the above diseases from GARDASIL.
What important information about GARDASIL should I know?
- You should continue to get routine cervical cancer screening.
- GARDASIL may not fully protect everyone who gets the vaccine.
- GARDASIL will not protect against HPV types that you already have.
- You may still benefit from GARDASIL if you do not already have all four vaccine types of HPV.
Who should not get GARDASIL?
You should not get GARDASIL if you have, or have had:
- an allergic reaction after getting a dose of GARDASIL.
- a severe allergic reaction to yeast, amorphous aluminum hydroxyphosphate sulfate, polysorbate 80.
What should I tell my health care provider before getting GARDASIL?
Tell your health care provider if you:
- are pregnant or planning to get pregnant. GARDASIL is not recommended for use in pregnant women.
- have immune problems, like HIV infection, cancer, or you take medicines that affect your immune system.
- have a fever over 100°F (37.8°C).
- had an allergic reaction to another dose of GARDASIL.
- take any medicines, even those you can buy over the counter.
Your health care provider will help decide if you should get the vaccine.
How is GARDASIL given?
GARDASIL is a shot that is usually given in the arm muscle. You will need 3 shots given on the following schedule:
- Dose 1: at a date you and your health care provider choose.
- Dose 2: 2 months after Dose 1.
- Dose 3: 6 months after Dose 1.
Fainting sometimes happens after getting GARDASIL. For this reason, your health care provider may ask you to sit or lie down for 15 minutes after you get GARDASIL.
Sometimes fainting has been accompanied by falling with injury, as well as shaking or stiffening and other seizure-like activity. This may require evaluation or treatment by your health care provider.
Make sure that you get all 3 doses on time so that you get the best protection. If you miss a dose, talk to your health care provider.
What are the possible side effects of GARDASIL?
The most common side effects with GARDASIL are:
- pain, swelling, itching, bruising, and redness at the injection site
- headache
- fever
- nausea
- dizziness
- vomiting
- fainting
Tell your health care provider if you have any of the following problems because these may be signs of an allergic reaction:
- difficulty breathing
- wheezing (bronchospasm)
- hives
- rash
Tell your health care provider if you have:
- swollen glands (neck, armpit, or groin)
- joint pain
- unusual tiredness or weakness
- chills
- generally feeling unwell
- leg pain
- shortness of breath
- chest pain
- aching muscles
- muscle weakness
- seizure
- bad stomach ache
Contact your health care provider right away if you get any symptoms that concern you, even several months after getting the vaccine.
For a more complete list of side effects, ask your health care provider.
What are the ingredients in GARDASIL?
The ingredients are proteins of HPV Types 6, 11, 16, and 18, amorphous aluminum hydroxyphosphate sulfate, yeast protein, sodium chloride, L-histidine, polysorbate 80, sodium borate, and water for injection.
This leaflet is a summary of information about GARDASIL. If you would like more information, please talk to your health care provider or visit www.gardasil.com.
Manufactured and Distributed by:
MERCK & CO., Inc.
Whitehouse Station, NJ 08889, USA
Issued June 2009
9883611
This is a representative sample of the packaging. Not inclusive of the labeling.
PRINCIPAL DISPLAY PANEL - LABEL - 1 DOSE VIAL 0.5 mL
1 DOSE VIAL 0.5 mL
[Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant]
GARDASIL®
U.S. Govt. Lic. No. 2
Rx only
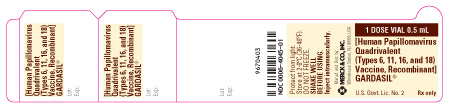
PRINCIPAL DISPLAY PANEL - CARTON - 1 SINGLE-DOSE VIAL 0.5 mL
NDC 0006-4045-00
1 Single-dose Vial 0.5 mL
REFRIGERATE
[Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant]
GARDASIL®
0.5 mL dose contains human papillomavirus L1 protein of each type adsorbed on amorphous aluminum hydroxyphosphate sulfate adjuvant as follows: Type 6 (20 mcg), Type 11 (40 mcg), Type 16 (40 mcg), Type 18 (20 mcg)
Vaccine is prepared from fermentation cultures of a recombinant strain of yeast Saccharomyces cerevisiae containing the genes for the human papillomavirus L1 protein of each type (6, 11, 16, 18).
Contains no preservative or antibiotics.
Rx only
MERCK
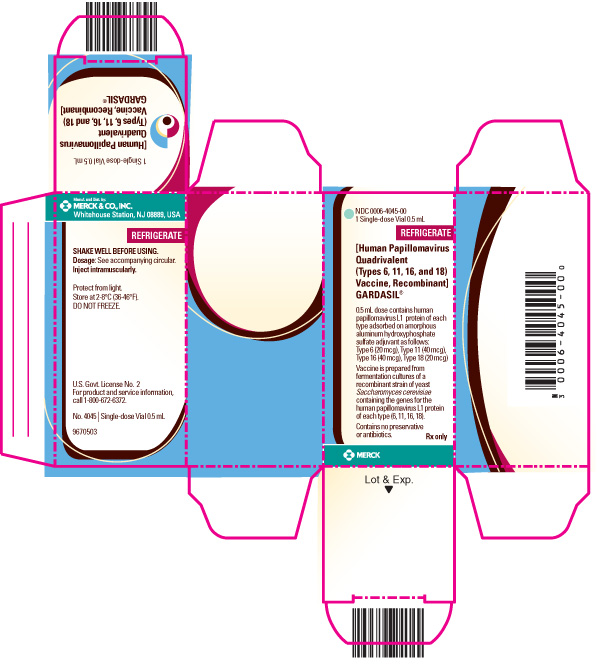
PRINCIPAL DISPLAY PANEL - LABEL - 1 DOSE SYRINGE 0.5 mL
1 DOSE SYRINGE 0.5 mL
[Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant]
GARDASIL®
Protect from light.
Store at 2-8°C (36-46°F).
DO NOT FREEZE.
SHAKE WELL BEFORE USING.
Inject intramuscularly.
U.S. Govt. Lic. No. 2
Rx only

PRINCIPAL DISPLAY PANEL - CARTON - 6 SINGLE-DOSE 0.5 mL SYRINGES WITH SEPARATE 1" NEEDLES
MERCK
NDC 0006-4109-06
6 Single-dose 0.5 mL Syringes With Separate 1” Needles
REFRIGERATE
[Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant]
GARDASIL®
Each 0.5 mL dose contains human papillomavirus L1 protein of each type adsorbed on amorphous aluminum hydroxyphosphate sulfate adjuvant as follows:
Type 6 (20 mcg), Type 11 (40 mcg), Type 16 (40 mcg), Type 18 (20 mcg)
Vaccine is prepared from fermentation cultures of a recombinant strain of yeast Saccharomyces cerevisiae containing the genes for the human papillomavirus L1 protein of each type (6, 11, 16, 18).
Contains no preservative or antibiotics.
Rx only
| GARDASIL
human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant injection, suspension |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA125126 | 06/08/2006 | |
| GARDASIL
human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant injection, suspension |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA125126 | 06/08/2006 | |
| Labeler - Merck & Co., Inc. (001317064) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Merck & Co., Inc. | 002387926 | MANUFACTURE | |