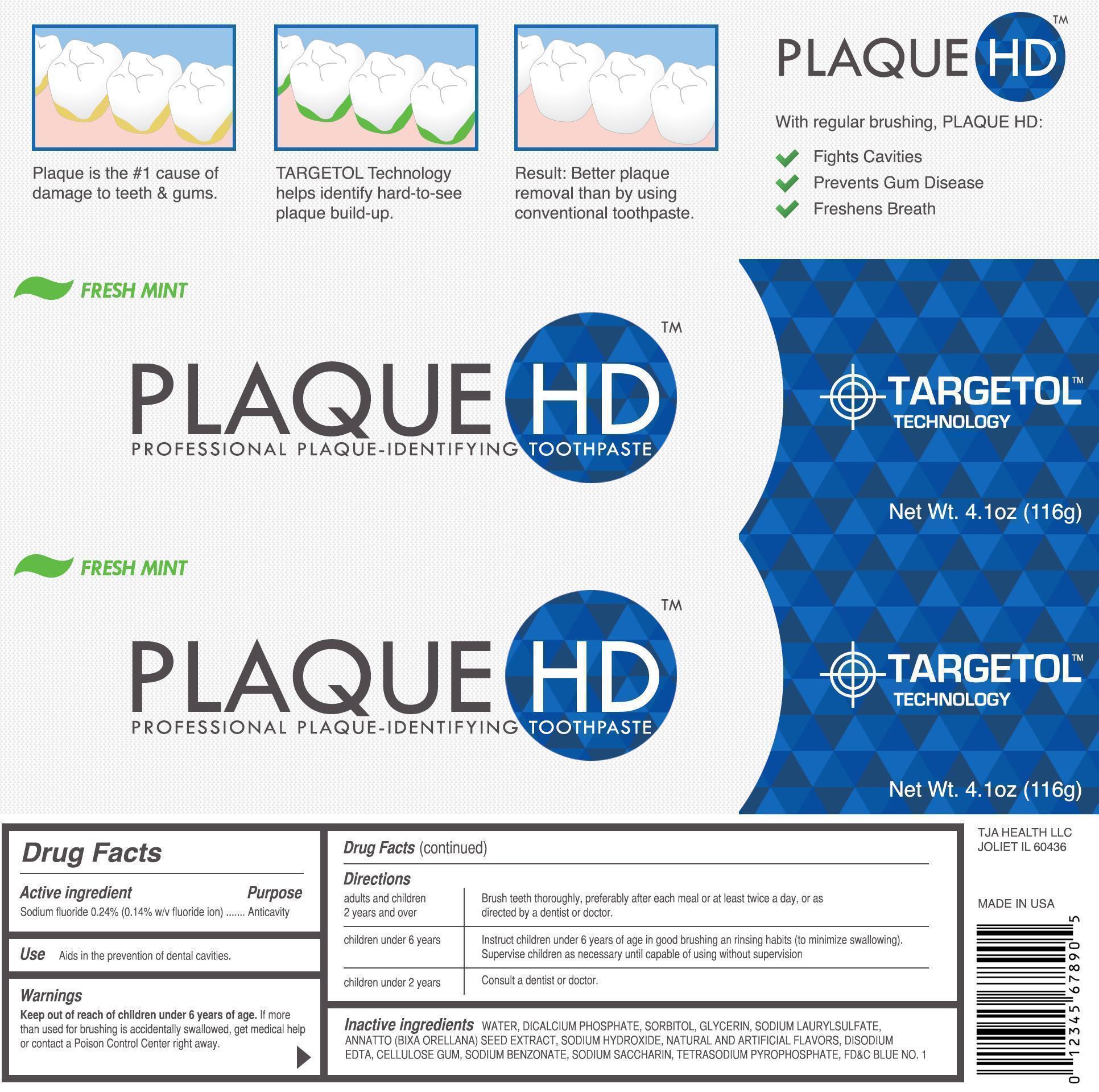
FRESH MINT PLAQUE HD PROFESSIONAL PLAQUE IDENTIFYING- sodium fluoride paste, dentifrice
TJA Health LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Plaque HD
Directions
| adults and children 2 years and over | Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor. |
| children under 6 years of age | Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing). Supervise children as necessary until capable of using without a supervision. |
| children under 2 years of age | Consult a dentist or doctor. |
Inactive Ingredients
WATER, DICALCIUM PHOSHATE, SORBITOL, GLYCERIN, TETRASODIUM PYROPHOSPHATE, CELLULOSE GUM, SODIUM BENZOATE, SODIUM LAURYL SULFATE, DISODIUMM EDTA, CARBOMER, SODIUM SACCHARIN, NATURAL AND ARTIFICIAL FLAVORS, ANNATTO ( BIXA ORELLANA) SEED EXTRACT, FD AND C BLUE NO.1
| FRESH MINT PLAQUE HD PROFESSIONAL PLAQUE IDENTIFYING
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - TJA Health LLC (078799634) |
| Registrant - TJA Health LLC (078799634) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TJA Health LLC | 078799634 | manufacture(57660-000) | |
Revised: 2/2014
Document Id: b091e5e2-843f-4db4-91b7-653c2bc4fd85
Set id: 98d07260-5274-42b7-bbf8-9683c5e8b624
Version: 7
Effective Time: 20140206
TJA Health LLC