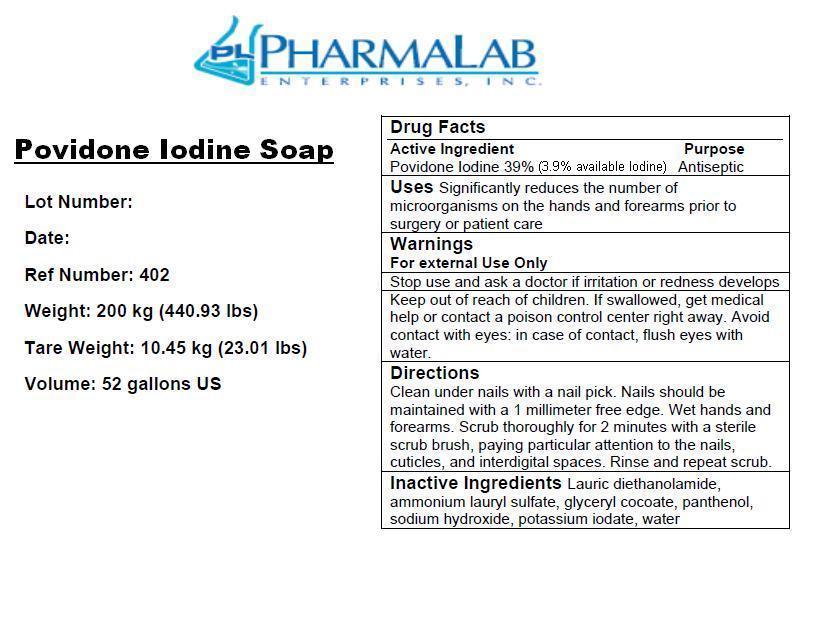
IODOPHOR SURGICAL HAND SCRUB- povidone-iodine soap
Pharmalab Enterprises Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUM LABEL
Uses: Significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care.
Inactive Ingredients:Lauric Diethanolamide, Ammonium Lauryl Sulfate, Glyceryl Cocoate, Panthenol, Sodium Hyrdoxide, Potassium Iodate, Water
Directions:
Clean under the nails with a nail pick. Nails should be maintained with a 1 millimeter free edge. Wet hands and forearms. Apply a palmful (5g) of foam to hands and forearms. Scrub thoroughly for 2 minutes with a sterile scrub brush, paying particular attention to the nails, cuticles, and interdigital spaces. Rinse and repeat scrub.
| IODOPHOR
SURGICAL HAND SCRUB
povine-iodine soap |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Pharmalab Enterprises Inc. (174401088) |