FLEBOGAMMA- human immunoglobulin g injection, solution
GRIFOLS USA, LLC
----------
Immune Globulin Intravenous (Human)
Flebogamma® 5%
DESCRIPTION
Immune Globulin Intravenous (Human), Flebogamma® 5% (IGIV) is a sterile, clear or slightly opalescent and colorless to pale yellow, liquid, pasteurized preparation of highly purified immunoglobulin (IgG) obtained from human plasma pools. The purification process includes cold alcohol fractionation, polyethylene glycol precipitation, and ion exchange chromatography.
Flebogamma® 5% is a highly purified (≥ 99% IgG), unmodified, human IgG that contains the antibody specificities found in the donor population. IgG subclasses are fully represented with the following approximate percents of total IgG: IgG1, is 70.3%, IgG2, 24.7%, IgG3, 3.1%, and IgG4, 1.9% (1). The IgA content is < 0.05 mg/mL, and IgM is present in trace amounts.
In the final formulation, Flebogamma® 5% contains 50 mg IgG per mL, 50 mg D-sorbitol per mL, and ≤ 6 mg/mL polyethylene glycol. There is no preservative in the formulation. The pH of the solution ranges from 5 to 6 and the osmolarity from 240 to 350 mOsm/L.
All Source Plasma used in the manufacture of this product was tested by FDA-licensed serological tests for HBsAg, antibodies to HCV and HIV and Nucleic Acid Test (NAT) for HCV and HIV-1 and found to be nonreactive (negative).
Virus elimination experiments have been performed on 2 steps of the production process. Residual viral titers were determined by infectivity assays. When no residual virus was detected, the Poisson distribution was used to give the minimum detectable level (MDL) based on the assay sensitivity and the sample volume used (1).
The viral reduction data (in log10) from these experiments are summarized in Table 1.
| Target Virus | HIVa | HBV, Herpesvirus | HCV | HAV | Parvovirus B19 | ||
|---|---|---|---|---|---|---|---|
| Model | HIV-1 | IBR | PRV | BVDV | Sindbis | EMC | PPV |
|
a Abbreviations: HIV = Human immunodeficiency virus; HBV = Hepatitis B virus; HCV = Hepatitis C virus; HAV = Hepatitis A virus; IBR = Infectious Bovine Rhinotracheitis virus; PRV = Pseudorabies virus; BVDV = Bovine Viral Diarrhoea virus; EMC = Encephalomyocarditis virus; PPV = Porcine Parvovirus. |
|||||||
| Pasteurization (60°C, 10 h) | > 5.9 | 5.8 | ≥ 4.3 | ≥ 5.1 | ≥ 6.6 | 5.0 | 2.3 |
| PEG precipitation | 3.7 | 4.3 | 4.2 | ≥ 5.3 | 4.2 | 3.8 | 3.9 |
| Cumulative | ≥ 9.6 | 10.1 | ≥ 8.5 | ≥ 10.4 | ≥ 10.8 | 8.8 | 6.2 |
CLINICAL PHARMACOLOGY
Flebogamma® 5% was administered as an IV infusion (300 to 600 mg/kg) to subjects with primary humoral immunodeficiency disease (PID) every 3 (n = 11) or 4 (n = 10) weeks for 12 months. The pharmacokinetics of total IgG was determined after the 7th infusion for the 3-week dosing interval and after the 5th infusion for the 4-week dosing interval (Table 2).
| Variable | 3-Week Dosing Interval | 4-Week Dosing Interval | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
|
a The numbers in brackets are the minimum and maximum values. |
||||
|
b For a subject on the 3-week schedule, the average of the trough levels from Infusion 7 to the end of the study was calculated; for those on a 4-week schedule, the average of the trough levels from Infusion 5 to the end of the study was calculated. The means of the subject means are presented in this table. |
||||
| Cmax (mg/dL) | 1845 | 389 | 1900 | 277 |
| [1340 - 2430]a | [1490 - 2430] | |||
| AUC0 - ∞ (day·mg/dL) | 63388 | 29583 | 91337 | 35915 |
| [18570 - 119909] | [48360 - 161073] | |||
| Clearance (mL/day) | 70 | 58 | 40 | 21 |
| [23 - 177] | [20 - 78] | |||
| Half-life (days) | 30 | 12 | 45 | 17 |
| [13 - 54] | [23 - 75] | |||
| Trough IgG level (mg/dL)b | 832.7 | 822.2 | 870.4 | 856.3 |
| [317.4 - 1207.5] | [660.0 - 1111.0] | |||
Pharmacokinetic data for antibodies to specific antigens are in Table 3.
| Test (unit) | Statistic | 3-Week Dosing Interval | 4-Week Dosing Interval | ||||
|---|---|---|---|---|---|---|---|
| Cmax
(mg/dL) | Trough (mg/dL) | Half-life (days) | Cmax
(mg/dL) | Trough (mg/dL) | Half-life (days |
||
| CMV IgG (IV) | Mean (SD) | 30 (36) | 11 (16) | 22 (9) | 30 (10) | 12 (8) | 30 (8) |
| Min - Max | 14 - 138 | 3 - 58 | 13 - 38 | 18 - 48 | 5 - 25 | 21 - 45 | |
| S. pneumoniae | Mean (SD) | 12 (4) | 6 (4) | 23 (8) | 13 (4) | 6 (2) | 29 (4) |
| Type 14 (μg/mL) | Min – Max | 7 - 21 | 2 - 18 | 14 - 33 | 9 - 22 | 3 - 10 | 22 - 33 |
| S. pneumoniae | Mean (SD) | 14 (12) | 5 (7) | 41 (39) | 9 (2) | 4 (1) | 25 (6) |
| Type 19F (μg/mL) | Min – Max | 6 - 43 | 1 - 25 | 11 - 132 | 7 - 13 | 2 - 6 | 16 - 36 |
| S. pneumoniae | Mean (SD) | 2 (0.5) | 1 (0.4) | 50 (77) | 2 (1) | 1 (1) | 43 (24) |
| Type 4 (μg/mL) | Min – Max | 1 - 2 | 0 - 2 | 10 - 254 | 1 - 4 | 0 - 2 | 21 - 82 |
| S. pneumoniae | Mean (SD) | 9 (2) | 4 (2) | 29 (21) | 9 (3) | 4 (1) | 36 (22) |
| Type 6B (μg/mL) | Min – Max | 6 - 13 | 1 - 9 | 13 - 73 | 7 - 15 | 2 - 7 | 21 - 86 |
| S. pneumoniae | Mean (SD) | 12 (6) | 4 (2) | 45 (60) | 11 (4) | 4 (1) | 42 (42) |
| Type 9V (μg/mL) | Min – Max | 6 - 25 | 1 - 8 | 11 - 170 | 8 - 18 | 2 - 6 | 17 - 143 |
| Tetanus Antitoxoid | Mean (SD) | 12 (2) | 5 (2) | 23 (11) | 14 (3) | 5 (1) | 28 (11) |
| Antibody (IU/mL) | Min - Max | 9 - 16 | 2 - 8 | 11 - 45 | 10 - 18 | 3 - 6 | 13 - 41 |
There is evidence that the half-life of IgG can vary considerable among patients (2-5).
There were 2 adolescent (≤ 16 years of age) subjects who underwent pharmacokinetic testing, and both of them were on the 3-week infusion schedule. There were no clinically relevant differences among the adults and adolescents that were tested.
Clinical:
Grifols-04-I was a multicenter, open-label, historically controlled study conducted in the United States. A total of 51 subjects were enrolled, and their data were analyzed for safety and efficacy. The primary efficacy variable was the number of episodes of the following serious infections: bacterial pneumonia, bacteremia or sepsis, osteomyelitis/septic arthritis, visceral abscesses and bacterial meningitis. The secondary efficacy variables were the number of days of work/school missed, the number of hospitalizations and the number of days of each hospitalization, the number of visits to physicians or emergency rooms, and the number of other infections documented by positive radiographic findings and fever.
The results showed that subjects had a serious infection rate of 0.061 infections/subject/year (98% confidence interval = 0.011 to 0.183), a rate that is much less than 1 infection/subject/year. With regard to the secondary efficacy variables, the mean rate was less than 10 days or visits/subject/year (Table 4), and there were no other infections documented by positive radiographic findings and fever.
| Variable | Subjects | Total Days or Visits | Total Subject Years | Days or Visits/Subject/Year | ||
|---|---|---|---|---|---|---|
| N | % | Estimatea | 95% C. I.b | |||
|
a Estimate = Total days or visits/total subject years. |
||||||
|
b The 95% confidence intervals were obtained by using a generalized linear model procedure for Poisson distribution. |
||||||
| Work/School Days Missed | 22 | 43 | 328 | 50.8 | 6.46 | 5.69, 7.29 |
| Days in Hospital | 9 | 18 | 54 | 50.9 | 1.1 | 0.77, 1.42 |
| Visits to Physician/ER | 40 | 78 | 201 | 50.9 | 3.95 | 3.36, 4.61 |
The dosing statistics for this study are in Table 5.
| Statistic | 3-Week Dosing Interval | 4-Week Dosing Interval | Total |
|---|---|---|---|
|
a Q1 is the 25th percentile, and Q3 is the 75th percentile. |
|||
| n | 15 | 36 | 51 |
| Mean (SD) | 437.5 (92.07) | 427.0 (78.44) | 430.1 (81.88) |
| Median | 442.8 | 432.3 | 436.2 |
| Q1, Q3a | 378.8, 480.8 | 367.9, 498.4 | 375.5, 497.0 |
| Min, Max | 307.0, 609.4 | 248.4, 572.4 | 248.4, 609.4 |
INDICATIONS AND USAGE
Flebogamma® 5% is indicated for replacement therapy in primary (inherited) humoral immunodeficiency disorders, such as common variable immunodeficiency, x-linked agammaglobulinemia, severe combined immunodeficiency, and Wiskott-Aldrich Syndrome. Flebogamma® 5% is especially useful when rapid replacement of IgG or the attainment of high serum levels of IgG is desired.
Some clinical trials conducted with Flebogamma® 5% included infants, children, and adolescents with primary and secondary immunodeficiency diseases to assess clinical efficacy. Data have also been obtained from postmarketing studies and postmarketing surveillance. Clinical trials with Flebogamma® 5% enrolled a very limited number of children and adolescents with primary humoral immune deficiency, a number insufficient to fully characterize the efficacy and safety in pediatric patients [See PRECAUTIONS, Pediatric Use].
CONTRAINDICATIONS
Flebogamma® 5% should not be administered to individuals with a history of severe or anaphylactic reactions to blood or blood-derived products. Individuals with selective IgA deficiency and demonstrable antibodies to IgA should not receive Flebogamma® 5%. If patients are known to be intolerant to any component of Flebogamma® 5%, such as sorbitol (i.e., intolerance to fructose), they should not receive the product.
WARNINGS
|
Immune Globulin Intravenous (Human) (IGIV) products have been reported to be associated with renal dysfunction, acute renal failure, osmotic nephrosis, and death (6). Patients predisposed to acute renal failure include patients with any degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Especially in such patients, IGIV products should be administered at the minimum concentration available and the minimum rate of infusion practicable. While these reports of renal dysfunction and acute renal failure have been associated with the use of many of the licensed IGIV products, those containing sucrose as a stabilizer accounted for a disproportionate share of the total number. Flebogamma® 5% does not contain sucrose. |
|
See PRECAUTIONS and DOSAGE AND ADMINISTRATION sections for important information intended to reduce the risk of acute renal failure. |
Flebogamma® 5% is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, and theoretically, the Creutzfeldt-Jakob (CJD) agent that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses. (See DESCRIPTION section). Plasma pools for manufacture are screened using Nucleic Acid Testing with polymerase chain reaction technology for HIV-1 and HCV. Two steps in the manufacturing process, Pasteurization for 10 hours at 60 degrees centigrade and Polyethylene Glycol precipitation, have been evaluated and demonstrated to provide cumulative log reductions exceeding 6 logs for all the tested viruses. Despite these measures, such IGIV products can still potentially transmit disease. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Grifols Biologicals at 888-GRIFOLS (888-474-3657). Physicians should discuss the potential risks and benefits of the use of this product with the patient.
All patients, but especially individuals receiving Flebogamma® 5% for the first time or being restarted on the product after a treatment hiatus of more than 8 weeks, may be at risk for the development of inflammatory reactions characterized by fever, chills, nausea, and vomiting. Careful monitoring of recipients and adherence to recommendations regarding information in the DOSAGE AND ADMINISTRATION section may reduce the risk of these types of events.
Appropriate supportive care, including immediate access to epinephrine injection, should be available for the management of acute anaphylactic reactions.
PRECAUTIONS
General:
Any vial that has been entered should be used promptly. Partially used vials should be discarded and not saved for future use because the solution contains no preservative. Do not use if turbid. Solution that has been frozen should not be used.
Ensure that patients are not volume-depleted before the initiation of the infusion of IGIV.
Renal Function:
Periodic monitoring of renal function and urine output is particularly important in patients judged to have a potential increased risk for developing acute renal failure (6). Renal function, including measurement of blood urea nitrogen (BUN)/serum creatinine, should be assessed before the initial infusion of Flebogamma® 5% and again at appropriate intervals thereafter. If renal function deteriorates, discontinuation of the product should be considered.
For patients judged to be at risk for developing renal dysfunction, it may be prudent to reduce the amount of product infused per unit time by infusing Flebogamma® 5% at a maximum rate less than 0.06 mL/kg (3 mg/kg) body weight/minute.
Aseptic Meningitis Syndrome:
An aseptic meningitis syndrome (AMS) has been reported to occur infrequently in association with IGIV treatment. The syndrome usually begins within several hours to 2 days following IGIV treatment. It is characterized by symptoms and signs including severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, and nausea and vomiting. Cerebrospinal fluid (CSF) studies are frequently positive with pleocytosis up to several thousand cells per cubic milliliter, predominantly from the granulocytic series, and with elevated protein levels up to several hundred mg/dL. Patients exhibiting such symptoms and signs should receive a thorough neurological examination, including CSF studies, to rule out other causes of meningitis. AMS may occur more frequently in association with high-dose (e.g., >1.0 g/kg body weight) and/or rapid-infusion IGIV treatment. Discontinuation of IGIV treatment has resulted in remission of AMS within several days without sequelae (7-10).
Hemolysis:
Immune Globulin Intravenous (Human) (IGIV) products can contain blood group antibodies which may act as hemolysins and induce in vivo coating of red blood cells with immunoglobulin, causing a positive direct antiglobulin reaction and, rarely, hemolysis (11-13). Hemolytic anemia can develop subsequent to IGIV therapy due to enhanced RBC sequestration (14) [See ADVERSE REACTIONS]. IGIV recipients should be monitored for clinical signs and symptoms of hemolysis [See PRECAUTIONS: Laboratory Tests].
Thrombotic Events:
Thrombotic events have been reported in association with IGIV (15-17) (See ADVERSE REACTIONS). Patients at risk may include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, and/or known or suspected hyperviscosity. The potential risks and benefits of IGIV should be weighed against those of alternative therapies for all patients for whom IGIV administration is being considered. Baseline assessment of blood viscosity should be considered in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies [See PRECAUTIONS: Laboratory Tests].
Transfusion-Related Acute Lung Injury (TRALI):
There have been reports of noncardiogenic pulmonary edema [Transfusion-Related Acute Lung Injury (TRALI)] in patients administered IGIV (18). TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever and typically occurs within 1 to 6 hours after transfusion. Patients with TRALI may be managed by using oxygen therapy with adequate ventilatory support.
IGIV recipients should be monitored for pulmonary adverse reactions. If TRALI is suspected, appropriate tests should be performed for the presence of antineutrophil antibodies in both the product and patient serum [See PRECAUTIONS: Laboratory Tests].
Information for Patients:
Patients should be instructed to immediately report symptoms of decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath (which may suggest kidney damage) to their physicians.
It is recommended that the lot number of the vials used be recorded when Flebogamma® 5% is administered.
Laboratory Tests:
Renal function, including measurement of blood urea nitrogen (BUN)/serum creatinine, should be assessed before the initial infusion of Flebogamma® 5% in patients judged to have a potential increased risk for developing acute renal failure and again at appropriate intervals thereafter.
Following infusion of Flebogamma® 5%, there may be a transitory rise of various antibody titers that may result in misleading positive results in serological testing.
Baseline assessment of blood viscosity should be considered in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies.
If TRALI is suspected, appropriate tests should be performed for the presence of antineutrophil antibodies in both the product and patient serum.
Pregnancy Category C:
Animal reproduction studies have not been performed with Flebogamma® 5%. It is also not known whether Flebogamma® 5% can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Flebogamma® 5% should be given to a pregnant woman only if clearly needed.
Drug Interactions:
Antibodies in Flebogamma® 5% may interfere with the response to live viral vaccines, such as measles, mumps, and rubella. Physicians should be informed of recent therapy with Immune Globulin Intravenous (Human) so that administration of live viral vaccines, if indicated, can be appropriately delayed 3 or more months from the time of IGIV administration.
Pediatric Use:
Ninety-four pediatric subjects, including 15 who were diagnosed with primary humoral immunodeficiency, have received Flebogamma® 5% in the course of clinical studies or postmarketing studies over a 14-year period. Although preliminary safety data in children and adolescents with primary humoral immune deficiency who received Flebogamma® 5% or other Immune Globulin Intravenous (Human) products produced by the same manufacturing method in different manufacturing facilities from that used for the production of Flebogamma® 5% has not revealed differences between the safety profiles of the product(s) in pediatric and adult patients, the experience has been too limited to consider the safety and efficacy of Flebogamma® 5% to be established in children and adolescents.
ADVERSE REACTIONS
Increases of creatinine and blood urea nitrogen (BUN) have been observed as soon as 1 to 2 days following infusion of IGIV. Progression to oliguria and anuria requiring dialysis has been observed, although some patients have improved spontaneously following cessation of treatment (19). Types of severe renal adverse reactions that have been seen following IGIV therapy include: acute renal failure, acute tubular necrosis (20), proximal tubular nephropathy, and osmotic nephrosis (6).
Certain severe adverse reactions may be related to the rate of infusion. The recommended infusion rate [See DOSAGE AND ADMINISTRATION] must be closely followed. Patients must be closely monitored and carefully observed for any symptoms throughout the infusion period. Adverse reactions may occur more frequently when a high infusion rate is used, the treatment is the initial exposure to immunoglobulin, the immunoglobulin product has been changed to that of a different manufacturer, or there has been a long interval (more than 8 weeks) since the previous infusion. Slowing or stopping an infusion usually results in the prompt disappearance of symptoms.
Postmarketing:
The following adverse reactions have been identified and reported during the post-approval use of IGIV products (21).
| Respiratory | Apnea, Acute Respiratory Distress Syndrome (ARDS), Transfusion-Related Acute Lung Injury (TRALI), cyanosis, hypoxemia, pulmonary edema, dyspnea, bronchospasm |
| Cardiovascular | Cardiac arrest, thromboembolism, vascular collapse, hypotension |
| Neurological | Coma, loss of consciousness, seizures, tremor |
| Integumentary | Stevens-Johnson Syndrome, epidermolysis, erythema multiformae, bullous dermatitis |
| Hematologic | Pancytopenia, leukopenia, hemolysis, positive direct antiglobulin (Coombs) test |
| General/Body as a Whole | Pyrexia, rigors |
| Musculoskeletal | Back pain |
| Gastrointestinal | Hepatic dysfunction, abdominal pain |
Because postmarketing reporting of these reactions is voluntary and the at-risk populations are of uncertain size, it is not always possible to reliably estimate the frequency of the reaction or establish a causal relationship to exposure to the product. Such is also the case with literature reports authored independently.
Adverse events were reported in a study of 51 individuals with primary humoral immunodeficiency diseases receiving infusions every 3 to 4 weeks of 300 to 600 mg/kg body weight. Forty-nine (96%) subjects experienced at least 1 adverse event irrespective of the relationship with the product, and these subjects reported a total of 784 adverse events. None of the 51 subjects who participated in this study discontinued the study prematurely due to an adverse experience.
Adverse events that occurred with an incidence of > 15% on a per subject basis are summarized in Table 6. No adverse events occurred with an incidence of > 2% on a per infusion basis.
| Adverse Event | Number of AEs | Number of Subjects with AE | Percent of Subjects with AE |
|---|---|---|---|
|
a NOS = not otherwise specified. |
|||
| Bronchitis | 17 | 10 | 20 |
| Cough and productive cough | 26 | 13 | 25 |
| Diarrhoea NOSa | 16 | 10 | 20 |
| Headache NOS and sinus headache | 61 | 27 | 53 |
| Nasal congestion | 22 | 11 | 21 |
| Pain NOS | 14 | 8 | 16 |
| Pyrexia | 31 | 14 | 27 |
| Rhinorrhoea | 16 | 10 | 20 |
| Sinusitis NOS | 43 | 20 | 39 |
| Sore throat NOS | 13 | 10 | 20 |
| Upper Respiratory tract infection | 22 | 17 | 33 |
| Wheezing and asthma aggravated | 24 | 9 | 18 |
Forty-six (90%) subjects had 331 adverse events that occurred during an infusion or within 72 hours after the completion of the infusion. Therefore 42% of all adverse events, regardless of assessed causality, were temporally associated with the infusion of Flebogamma® 5%.
Overall, 217 of 746 infusions (29%) were temporally associated with 1 or more adverse events occurring within 72 hours after an infusion, regardless of assessed relationship to treatment; the 1-sided, 95%, upper-bound confidence interval was 34%.
A summary of infusions with mild, moderate, and severe treatment-related adverse events is in Table 7.
| Severity of AE | No. Infusions with AE | Adjusted %a | Confidence Intervalb |
|---|---|---|---|
|
a Adjusted % = average of the % of infusions with a treatment-related adverse event for each individual subject. |
|||
|
b The 95% upper bound for the adjusted % of infusions for which at least 1 treatment-related adverse event was reported was derived by using the t-statistic. |
|||
| Mild | 49 | 6.2 | 8.4 |
| Moderate | 12 | 1.5 | 2.3 |
| Severe | 3 | 0.4 | 0.8 |
The number and percent of subjects with treatment-emergent rises in AST or ALT are in Table 8.
| Laboratory Test | n | % |
|---|---|---|
| Assessment Criteria | ||
|
a ULN = upper limit of normal. |
||
| AST | ||
| Above the ULNa | 22 | 43 |
| Above 3 x the ULN | 3 | 6 |
| ALT | ||
| Above the ULN | 16 | 31 |
| Above 3 x the ULN | 1 | 2 |
None of these subjects had a concomitant treatment-emergent rise in total bilirubin.
Reported adverse reactions with Flebogamma® 5% and other IGIV products include: headache, chills, fever, shaking, fatigue, malaise, anxiety, back pain, muscle cramps, abdominal cramps, blood pressure changes, chest tightness, palpitations, tachycardia, nausea, vomiting, cutaneous reactions, wheezing, rash, arthralgia, and edema, often beginning within 60 minutes of the start of the infusion.
Rarely, Immune Globulin Intravenous (Human) can induce a severe fall in blood pressure with anaphylactic reaction, even in patients who had tolerated previous treatment with IGIV. In the case of shock, the current medical standards for shock treatment should be implemented.
DOSAGE AND ADMINISTRATION
The usual dose of Flebogamma® 5% for replacement therapy in primary humoral immunodeficiency diseases is 300 to 600 mg/kg body weight administered every 3 to 4 weeks. Doses may be adjusted over time to achieve the desired trough IgG levels and clinical responses. No randomized controlled trial data are available to determine an optimum target trough serum IgG level.
The infusion of Flebogamma® 5% should be initiated at a rate of 0.01 mL/kg body weight/minute (0.5 mg/kg/minute). If, during the first 30 minutes, the patient does not experience any discomfort, the rate may be gradually increased to a maximum of 0.10 mL/kg/minute (5 mg/kg/minute).
For patients judged to be at risk for developing renal dysfunction, it may be prudent to limit the amount of product infused per unit time by infusing Flebogamma® 5% at a maximum rate less than 0.06 mL/kg body weight/minute (3 mg/kg/minute). No prospective data are available to identify a maximum safe dose, concentration, and rate of infusion in patients determined to be at increased risk of acute renal failure. In the absence of prospective data, recommended doses should not be exceeded, and the concentration and infusion rate should be the minimum level practicable. Reduction in dose, concentration, and/or rate of infusion in patients at risk of acute renal failure, which includes patients over 65 [See WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS] has been proposed in the literature in order to reduce the risk of acute renal failure (22).
Compatibility Issues:
Flebogamma® 5% should be inspected visually for particulate matter and color prior to administration, if particles are detected the vial shall not be used. Do not use if turbid. If large doses are to be administered, several vials of Flebogamma® 5% may be pooled into an empty sterile IV solution container by using aseptic technique. Dilution with IV fluids is not recommended. An in-line filter with a pore size of 15 to 20 microns is recommended for the infusion. Antibacterial filters (0.2 micron) may also be used, although they may slow infusions. Discard unused contents and administration devices after use.
Specific drug interactions and incompatibilities have not been studied. Flebogamma® 5% should be infused through a separate intravenous line. Do not add any medications or IV fluids to the Flebogamma® 5% infusion container. Do not mix IGIV products of different formulations or from different manufacturers.
HOW SUPPLIED
Flebogamma® 5% is supplied in the following vial sizes:
| NDC Number | Size | Grams IgG |
| 61953-0003-1 | 10 mL | 0.5 |
| 61953-0003-2 | 50 mL | 2.5 |
| 61953-0003-3 | 100 mL | 5 |
| 61953-0003-4 | 200 mL | 10 |
REFERENCES
- Data on file. Instituto Grifols, S.A.
- Waldmann TA, Storber W. Metabolism of immunoglobulins. Prog Allergy 1969; 13:1-110.
- Morrell A, Riesen W. Structure, function and catabolism of immunoglobulins. In: Nydegger UE, editor. Immunohemotherapy. London: Academic Press; 1981, p.17-26.
- Stiehm ER. Standard and special human immune serum globulins as therapeutic agents. Pediatrics 1979; 63:301-19.
- Buckley RH. Immunoglobulin replacement therapy: indications and contraindications for use and variable IgG levels achieved. In: Alving BM, Finlayson JS, editors. Immunoglobulins: characteristics and use of intravenous preparations. Washington DC: US Department of Health and Human Services; 1979, p 3-8.
- Cayco AV, Perazella MA, Hayslett JP. Renal insufficiency after intravenous immune globulin therapy: a report of two cases and an analysis of the literature. J Am Soc Nephrol 1997; 8:1788-94.
- Sekul EA, Cupler EJ, Dalakas MC. Aseptic meningitis associated with high-dose intravenous immunoglobulin therapy: frequency and risk factors. Ann Intern Med 1994;121:259-62.
- Kato E, Shindo S, Eto Y, et al. Administration of immune globulin associated with aseptic meningitis. JAMA 1988; 259:3269-71.
- Casteels-Van Daele M, Wijndaele L, Hanninck K, et al. Intravenous immune globulin and acute aseptic meningitis. N Engl J Med 1990;323:614-5.
- Scribner CL, Kapit RM, Phillips ET, et al. Aseptic meningitis and intravenous immunoglobulin therapy. Ann Intern Med 1994;121:305-6.
- Copelan EA, Strohm PL, Kennedy MS, et al. Hemolysis following intravenous immune globulin therapy. Transfusion 1986;26:410-2.
- Thomas MJ, Misbah SA, Chapel HM, et al. Hemolysis after high-dose intravenous Ig. Blood 1993;15:3789.
- Reinhart WH, Berchtold PE. Effect of high-dose intravenous immunoglobulin therapy on blood rheology. Lancet 1992;339:662-4.
- Kessary-Shoham H, Levy Y,Shoenfeld Y, et al. In vivo administration of intravenous immunoglobulin (IVIg) can lead to enhanced erythrocyte sequestration. J Autoimmun 1999;13:129-35.
- Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 1994;44:223-6.
- Woodruff RK, Grigg AP, Firkin FC, et al. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet 1986;ii:217-8.
- Wolberg AS, Kon RH, Monroe DM, et al. Coagulation factor XI is a contaminant in intravenous immunoglobulin preparations. Am J Hematol 2000;65:30-4.
- Rizk A, Gorson KC, Kenney L, et al. Transfusion-related acute lung injury after the infusion of IVIG. Transfusion 2001;41:264-8.
- Winward DB, Brophy MT. Acute renal failure after administration of intravenous immunoglobulin: review of the literature and case report. Pharmacotherapy 1995;15:765-72.
- Phillips AO. Renal failure and intravenous immunoglobulin. Clin Nephrol 1992;37:217.
- Pierce LR, Jain N. Risks associated with the use of intravenous immunoglobulin. Transfus Med Rev 2003;17:241-51.
- Tan E, Hajinazarian M, Bay W, et al. Acute renal failure resulting from intravenous immunoglobulin therapy. Arch Neurol 1993;50:137-9.
Manufactured by INSTITUTO GRIFOLS, S.A.
BARCELONA – SPAIN
U.S. License No. 1181
Distributed by GRIFOLS BIOLOGICALS, Inc.
LOS ANGELES - CA 90032
Phone: 888-GRIFOLS (888-474-3657)
Revised September 2004
3021013
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 10 ML VIAL
NDC 61953-0003-1
Immune Globulin Intravenous (Human)
Flebogamma® 5% 0.5 g
0.5 g in 10 mL Rx only.
Store at 2 – 25 °C (36 – 77 °F).
Manufactured by Instituto Grifols, S.A.
Barcelona – SPAIN
U.S. License No. 1181
Distributed by Grifols Biologicals, Inc.
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 50 ML VIAL
NDC 61953-0003-2
Immune Globulin Intravenous (Human)
Flebogamma® 5% 2.5 g
2.5 g in 50 mL Rx only.
Dosage and directions for administration, see package insert.
Store at 2 – 25 °C (36 – 77 °F). DO NOT USE IF TURBID. ONCE THE CONTAINER HAS BEEN ENTERED IT SHOULD BE USED PROMPTLY.
Manufactured by Instituto Grifols, S.A. Barcelona – SPAIN U.S. License No. 1181
Distributed by Grifols Biologicals, Inc. Los Angeles – CA 90032
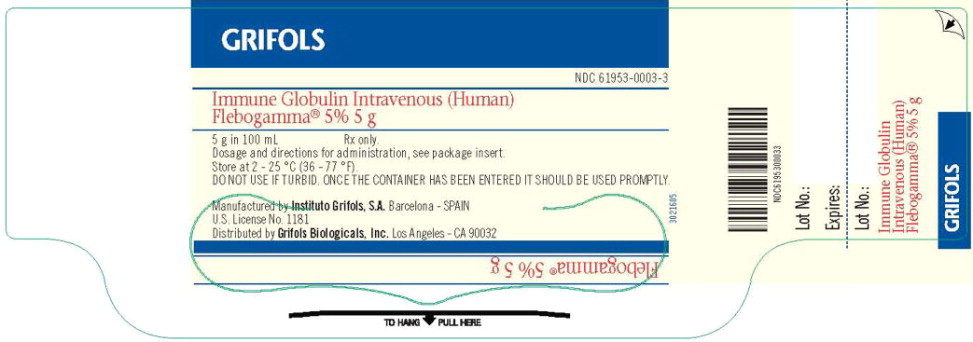
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 100 ML VIAL
GRIFOLS
NDC 61953-0003-3
Immune Globulin Intravenous (Human)
Flebogamma® 5% 5 g
5 g in 100 mL Rx only.
Dosage and directions for administration, see package insert.
Store at 2 – 25 °C (36 – 77 °F).
DO NOT USE IF TURBID. ONCE THE CONTAINER HAS BEEN ENTERED IT SHOULD BE USED PROMPTLY.
Manufactured by Instituto Grifols, S.A. Barcelona – SPAIN
U.S. License No. 1181
Distributed by Grifols Biologicals, Inc. Los Angeles – CA 90032
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 200 ML VIAL
GRIFOLS
NDC 61953-0003-4
Immune Globulin Intravenous (Human)
Flebogamma® 5% 10 g
10 g in 200 mL Rx only.
CONTENTS: Each 200 mL contains Immune Globulin (Human) 10 g; D-sorbitol 10 g; Protein contents is 50g/L
Dosage and directions for administration, see package insert. Store at 2 – 25 °C (36 – 77 °F).
DO NOT USE IF TURBID. ONCE THE CONTAINER HAS BEEN ENTERED IT SHOULD BE USED PROMPTLY.
The patient and physician should discuss risks and benefits of this product.
Manufactured by Instituto Grifols, S.A. Barcelona – SPAIN U.S. License No. 1181
Distributed by Grifols Biologicals, Inc. Los Angeles – CA 90032
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 10 ML CARTON
GRIFOLS
NDC 61953-0003-1
Immune Globulin Intravenous (Human)
Flebogamma® 5% 0.5 g
Solution for infusion
0.5 g in 10 mL
DO NOT USE IF TURBID.
ONCE THE CONTAINER HAS BEEN ENTERED IT SHOULD BE USED PROMPTLY.
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 50 ML CARTON
GRIFOLS
NDC 61953-0003-2
Immune Globulin Intravenous (Human)
Flebogamma® 5% 2.5 g
Solution for infusion
2.5 g in 50 mL
DO NOT USE IF TURBID.
ONCE THE CONTAINER HAS BEEN ENTERED IT SHOULD BE USED PROMPTLY.
| FLEBOGAMMA
immune globulin (human) injection, solution |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - GRIFOLS USA, LLC (048987452) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Instituto Grifols, S.A. | 465562213 | MANUFACTURE(61953-0003) | |