HYDROGEN PEROXIDE - hydrogen peroxide liquid
Medical Chemical Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
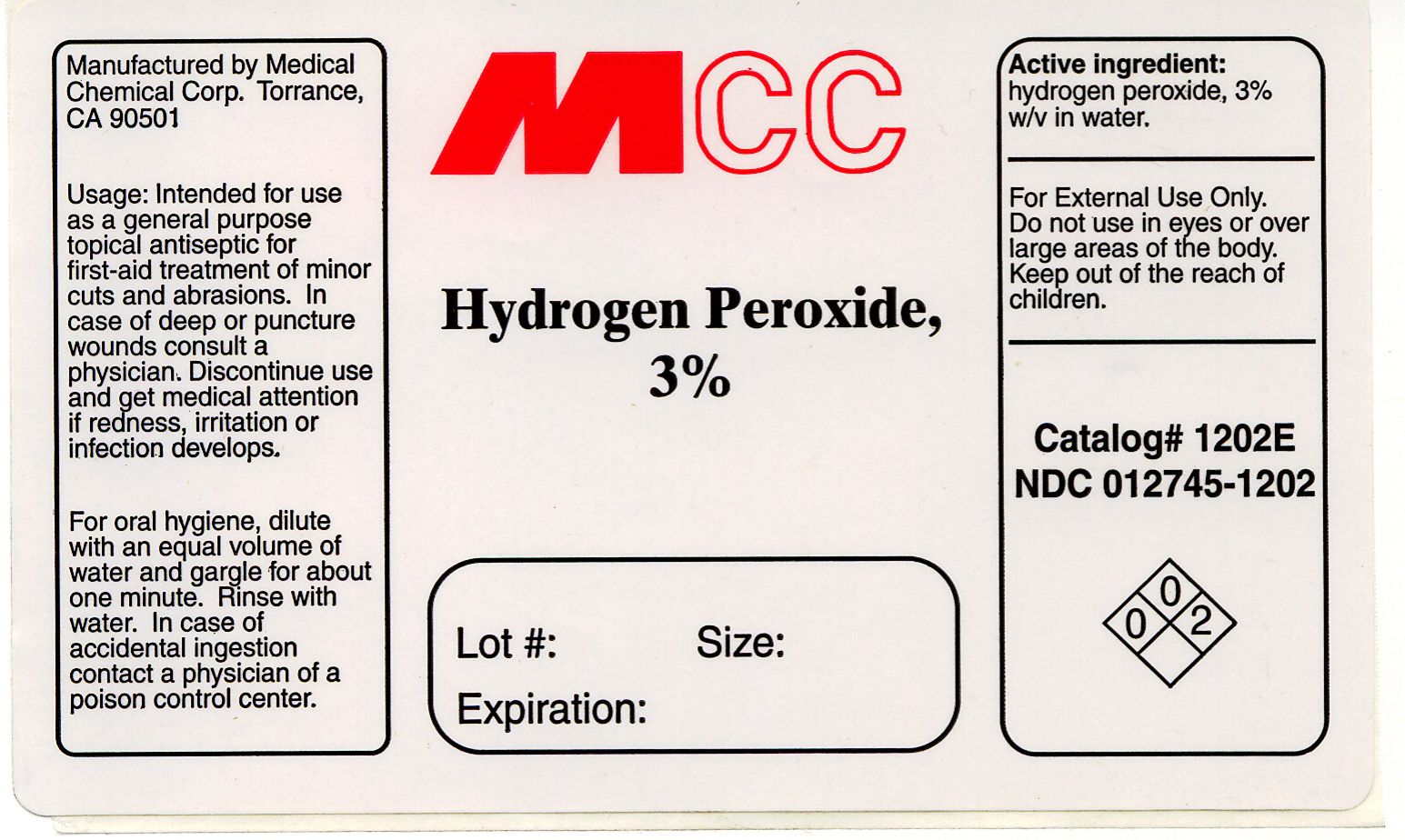
Hydrogen Peroxide 3% Label
Usage: Intended for use as a general purpose topical antiseptic for first-aid treatment of minor cuts and abrasions. In case of deep or puncture wounds consult a physician. Discontinue use and get medical attention if redness, irritation or infection develops.
For External Use Only. Do not use in eyes or over large areas of the body. Keep out of the reach of children.
Usage: Intended for use as a general purpose topical antiseptic for first-aid treatment of minor cuts and abrasions. In case of deep or puncture wounds consult a physician. Discontinue use and get medical attention if redness, irritation or infection develops.
For External Use Only. Do not use in eyes or over large areas of the body. Keep out of the reach of children.
| HYDROGEN PEROXIDE
hydrogen peroxide liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Medical Chemical Corporation (008496861) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Medical Chemical Corporation | 008496861 | manufacture(12745-202) | |