RHINOCORT AQUA
-
budesonide spray, metered
AstraZeneca LP
----------
RHINOCORT AQUA(budesonide)
Nasal Spray 32 mcg
DESCRIPTION
For Intranasal Use Only.
Budesonide, the active ingredient of RHINOCORT AQUA® Nasal Spray, is an anti-inflammatory synthetic corticosteroid.
It is designated chemically as (RS)-11-beta, 16-alpha, 17, 21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16, 17-acetal with butyraldehyde.
Budesonide is provided as the mixture of two epimers (22R and 22S).
The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5.
Its structural formula is:
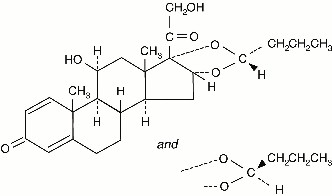
Budesonide is a white to off-white, odorless powder that is practically insoluble in water and in heptane, sparingly soluble in ethanol, and freely soluble in chloroform.
Its partition coefficient between octanol and water at pH 5 is 1.6 x 10.3
RHINOCORT AQUA is an unscented, metered-dose, manual-pump spray formulation containing a micronized suspension of budesonide in an aqueous medium. Microcrystalline cellulose and carboxymethyl cellulose sodium, dextrose anhydrous, polysorbate 80, disodium edetate, potassium sorbate, and purified water are contained in this medium; hydrochloric acid is added to adjust the pH to a target of 4.5.
RHINOCORT AQUA Nasal Spray delivers 32 mcg of budesonide per spray.
Each bottle of RHINOCORT AQUA Nasal Spray 32 mcg contains 120 metered sprays after initial priming.
Prior to initial use, the container must be shaken gently and the pump must be primed by actuating eight times. If used daily, the pump does not need to be reprimed. If not used for two consecutive days, reprime with one spray or until a fine spray appears. If not used for more than 14 days, rinse the applicator and reprime with two sprays or until a fine spray appears.
CLINICAL PHARMACOLOGY
Budesonide is a synthetic corticosteroid having potent glucocorticoid activity and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide has approximately a 200-fold higher affinity for the glucocorticoid receptor and a 1000-fold higher topical anti-inflammatory potency than cortisol (rat croton oil ear edema assay). As a measure of systemic activity, budesonide is 40 times more potent than cortisol when administered subcutaneously and 25 times more potent when administered orally in the rat thymus involution assay. In glucocorticoid receptor affinity studies, the 22R form was twice as active as the 22S epimer.
Inflammation is an important component in the pathogenesis of seasonal and perennial allergic rhinitis. Corticosteroids act on multiple sites of the inflammatory cascade resulting in a decrease of inflammation. Corticosteroids have been shown to have a wide range of inhibitory activities against multiple cell types (eg, mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (eg, histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic mediated inflammation.
Corticosteroids affect the delayed (6 hour) response to an allergen challenge more than the histamine-associated immediate response (20 minute). The clinical significance of these findings is unknown.
Pharmacokinetics
The pharmacokinetics of budesonide have been studied following nasal, oral, and intravenous administration. Budesonide is relatively well absorbed after both inhalation and oral administration, and is rapidly metabolized into metabolites with low corticosteroid potency. The clinical activity of RHINOCORT AQUA Nasal Spray is therefore believed to be due to the parent drug, budesonide. In vitro studies indicate that the two epimeric forms of budesonide do not interconvert.
Absorption
Following intranasal administration of RHINOCORT AQUA, the mean peak plasma concentration occurs at approximately 0.7 hours. Compared to an intravenous dose, approximately 34% of the delivered intranasal dose reaches the systemic circulation, most of which is absorbed through the nasal mucosa. While budesonide is well absorbed from the GI tract, the oral bioavailability of budesonide is low (~10%) primarily due to extensive first pass metabolism in the liver.
Distribution
Budesonide has a volume of distribution of approximately 2-3 L/kg. The volume of distribution for the 22R epimer is almost twice that of the 22S epimer. Protein binding of budesonide in vitro is constant (85-90%) over a concentration range (1-100 nmol/L), which exceeded that achieved after administration of recommended doses. Budesonide shows little to no binding to glucocorticosteroid binding globulin. It rapidly equilibrates with red blood cells in a concentration independent manner with a blood/plasma ratio of about 0.8.
Metabolism
Budesonide is rapidly and extensively metabolized in humans by the liver. Two major metabolites (16α-hydroxyprednisolone and 6β-hydroxybudesonide) are formed via cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4)-catalyzed biotransformation. Known metabolic inhibitors of CYP3A4 (eg, ketoconazole), or significant hepatic impairment, may increase the systemic exposure of unmetabolized budesonide (see WARNINGS and PRECAUTIONS). In vitro studies on the binding of the two primary metabolites to the glucocorticoid receptor indicate that they have less than 1% of the affinity for the receptor as the parent compound budesonide. In vitro studies have evaluated sites of metabolism and showed negligible metabolism in skin, lung, and serum. No qualitative difference between the in vitro and in vivo metabolic patterns could be detected.
Excretion/Elimination
Budesonide is excreted in the urine and feces in the form of metabolites. After intranasal administration of a radiolabeled dose, 2/3 of the radioactivity was found in the urine and the remainder in the feces. The main metabolites of budesonide in the 0-24 hour urine sample following IV administration are 16α-hydroxyprednisolone (24%) and 6β-hydroxybudesonide (5%). An additional 34% of the radioactivity recovered in the urine was identified as conjugates.
The 22R form was preferentially cleared with clearance value of 1.4 L/min vs. 1.0 L/min for the 22S form. The terminal half-life, 2 to 3 hours, was similar for both epimers and it appeared to be independent of dose.
Special Populations
Geriatric:
No specific pharmacokinetic study has been undertaken in subjects >65 years of age.
Pediatric:
After administration of RHINOCORT AQUA Nasal Spray, the time to reach peak drug concentrations and plasma half-life were similar in children and in adults. Children had plasma concentrations approximately twice those observed in adults due primarily to differences in weight between children and adults.
Gender:
No specific pharmacokinetic study has been conducted to evaluate the effect of gender on budesonide pharmacokinetics. However, following administration of 400 mcg of RHINOCORT AQUA Nasal Spray to 7 male and 8 female volunteers in a pharmacokinetic study, no major gender differences in the pharmacokinetic parameters were found.
Race:
No specific study has been undertaken to evaluate the effect of race on budesonide pharmacokinetics.
Renal Insufficiency:
The pharmacokinetics of budesonide have not been investigated in patients with renal insufficiency.
Hepatic Insufficiency:
Reduced liver function may affect the elimination of corticosteroids. The pharmacokinetics of orally administered budesonide were affected by compromised liver function as evidenced by a doubled systemic availability. The relevance of this finding to intranasally administered budesonide has not been established.
Nursing Mothers:
The disposition of budesonide when delivered by oral inhalation from a dry powder inhaler at doses of 200 or 400 mcg twice daily for at least 3 months was studied in eight lactating women with asthma from 1 to 6 months postpartum. Systemic exposure to budesonide in these women appears to be comparable to that in non-lactating women with asthma from other studies. Breast milk obtained over an 8–hour period post-dose revealed that the maximum concentration of budesonide for the 400 and 800 mcg total daily doses was 0.39 and 0.78 nmol/L, respectively, and occurred within 45 minutes after dosing. The estimated oral daily dose of budesonide from breast milk to the infant was approximately 0.007 and 0.014 mcg/kg/day for the two dose regimens used in this study, which represents approximately 0.3% to 1% of the dose inhaled by the mother. Budesonide levels in plasma samples obtained from five infants at about 90 minutes after breastfeeding (and about 140 minutes after drug administration to the mother) were below quantifiable levels (<0.02 nmol/L in four infants and <0.04 nmol/L in one infant) (see PRECAUTIONS, Nursing Mothers).
Pharmacodynamics
A 3-week clinical study in seasonal rhinitis, comparing RHINOCORT Nasal Inhaler, orally ingested budesonide, and placebo in 98 patients with allergic rhinitis due to birch pollen, demonstrated that the therapeutic effect of RHINOCORT Nasal Inhaler can be attributed to the topical effects of budesonide.
The effects of RHINOCORT AQUA Nasal Spray on adrenal function have been evaluated in several clinical trials. In a four-week clinical trial, 61 adult patients who received 256 mcg daily of RHINOCORT AQUA Nasal Spray demonstrated no significant differences from patients receiving placebo in plasma cortisol levels measured before and 60 minutes after 0.25 mg intramuscular cosyntropin. There were no consistent differences in 24-hour urinary cortisol measurements in patients receiving up to 400 mcg daily. Similar results were seen in a study of 150 children and adolescents aged 6 to 17 with perennial rhinitis who were treated with 256 mcg daily for up to 12 months.
After treatment with the recommended maximal daily dose of RHINOCORT AQUA (256 mcg) for seven days, there was a small, but statistically significant decrease in the area under the plasma cortisol-time curve over 24 hours (AUC0-24h) in healthy adult volunteers.
A dose-related suppression of 24-hour urinary cortisol excretion was observed after administration of RHINOCORT AQUA doses ranging from 100-800 mcg daily for up to four days in 78 healthy adult volunteers. The clinical relevance of these results is unknown.
Clinical Trials
The therapeutic efficacy of RHINOCORT AQUA Nasal Spray has been evaluated in placebo-controlled clinical trials of seasonal and perennial allergic rhinitis of 3-6 weeks duration.
The number of patients treated with budesonide in these studies was 90 males and 51 females aged 6-12 years and 691 males and 694 females 12 years and above. The patients were predominantly Caucasian.
Overall, the results of these clinical trials showed that RHINOCORT AQUA Nasal Spray administered once daily provides statistically significant reduction in the severity of nasal symptoms of seasonal and perennial allergic rhinitis including runny nose, sneezing, and nasal congestion.
An improvement in nasal symptoms may be noted in patients within 10 hours of first using RHINOCORT AQUA Nasal Spray. This time to onset is supported by an environmental exposure unit study in seasonal allergic rhinitis patients that demonstrated that RHINOCORT AQUA Nasal Spray led to a statistically significant improvement in nasal symptoms compared to placebo by 10 hours. Further support comes from a clinical study of patients with perennial allergic rhinitis which demonstrated a statistically significant improvement in nasal symptoms for both RHINOCORT AQUA Nasal Spray and for the active comparator (mometasone furoate) compared to placebo by 8 hours. Onset was also assessed in this study with peak nasal inspiratory flow rate and this endpoint failed to show efficacy for either active treatment. Although statistically significant improvements in nasal symptoms compared to placebo were noted within 8-10 hours in these studies, about one half to two thirds of the ultimate clinical improvement with RHINOCORT AQUA Nasal Spray occurs over the first 1-2 days, and maximum benefit may not be achieved until approximately 2 weeks after initiation of treatment.
INDICATIONS AND USAGE
RHINOCORT AQUA Nasal Spray is indicated for the management of nasal symptoms of seasonal or perennial allergic rhinitis in adults and children six years of age and older.
CONTRAINDICATIONS
Hypersensitivity to any of the ingredients in this preparation contraindicates the use of RHINOCORT AQUA Nasal Spray.
WARNINGS
The replacement of a systemic corticosteroid with a topical corticosteroid can be accompanied by signs of adrenal insufficiency, and in addition some patients may experience symptoms of corticosteroid withdrawal, e.g., joint and/or muscular pain, fatigue, weakness, nausea, vomiting, hypotension, lassitude, and depression. Patients previously treated for prolonged periods with systemic corticosteroids should be weaned off slowly when transferred to topical corticosteroids and carefully monitored for acute adrenal insufficiency in response to stress. In those patients who have asthma or other clinical conditions requiring long-term systemic corticosteroid treatment, too rapid a decrease in systemic corticosteroids may cause a severe exacerbation of their symptoms.
Transfer of patients from systemic corticosteroid therapy to RHINOCORT AQUA may unmask allergic or other immunologic conditions previously suppressed by the systemic corticosteroid therapy, e.g., rhinitis, conjunctivitis, eosinophilic conditions, eczema, and arthritis.
Patients who are on drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chicken pox, therapy with varicella zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG), as appropriate, may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information). If chicken pox develops, treatment with antiviral agents may be considered.
The clinical course of chicken pox or measles infection in patients on intranasal or inhaled corticosteroids has not been studied. While there is no data with intranasal corticosteroids, a clinical study has examined the immune responsiveness to the varicella vaccine in asthma patients 12 months to 8 years of age who were treated with budesonide inhalation suspension (see PRECAUTIONS, Drug Interactions).
Precautions
General
Intranasal corticosteroids, including budesonide may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth routinely of pediatric patients receiving long-term treatment with RHINOCORT AQUA. To minimize the systemic effects of intranasal corticosteroids, including RHINOCORT AQUA, titrate each patient’s dose to the lowest dosage that effectively controls his/her symptoms. (see PRECAUTIONS, Pediatric Use).
Rarely, immediate and/or delayed hypersensitivity reactions may occur after the intranasal administration of budesonide.
Rare instances of glaucoma, increased intraocular pressure, cataracts, wheezing and nasal septum perforation, and have been reported following the intranasal application of corticosteroids, including budesonide (see ADVERSE REACTIONS).
Although systemic effects have been minimal with recommended doses of RHINOCORT AQUA Nasal Spray, any such effect is dose dependent. Therefore, larger than recommended doses of RHINOCORT AQUA Nasal Spray should be avoided and the minimal effective dose for the patient should be used (see DOSAGE AND ADMINISTRATION). When used at larger doses, systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear. If such changes occur, the dosage of RHINOCORT AQUA Nasal Spray should be discontinued slowly, consistent with accepted procedures for discontinuing oral corticosteroid therapy.
In clinical studies with budesonide administered intranasally, the development of localized infections of the nose and pharynx with Candida albicans has occurred only rarely. When such an infection develops, it may require treatment with appropriate local or systemic therapy and discontinuation of treatment with RHINOCORT AQUA Nasal Spray. Patients using RHINOCORT AQUA Nasal Spray over several months or longer should be examined periodically for evidence of Candida infection or other signs of adverse effects on the nasal mucosa.
RHINOCORT AQUA Nasal Spray should be used with caution, if at all, in patients with active or quiescent tuberculous infection, untreated fungal, bacterial, or systemic viral infections, or ocular herpes simplex.
Because of the inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septal ulcers, nasal surgery, or nasal trauma should not use a nasal corticosteroid until healing has occurred.
Hepatic dysfunction influences the pharmacokinetics of budesonide, similar to the effect on other corticosteroids, with a reduced elimination rate and increased systemic availability (see CLINICAL PHARMACOLOGY, Special Populations).
Information for Patients
Patients being treated with RHINOCORT AQUA Nasal Spray should receive the following information and instructions.
This information is intended to aid the patient in the safe and effective use of the medication. It is not a disclosure of all possible adverse or intended effects. For proper use of RHINOCORT AQUA and to attain maximum improvement, the patient should read and follow the accompanying Patient Instructions for Use.
Patients should be warned to avoid exposure to chicken pox or measles and, if they are exposed, to consult their physicians without delay (see WARNINGS, and PRECAUTIONS, Drug Interactions).
Patients should use RHINOCORT AQUA Nasal Spray at regular intervals since its effectiveness depends on its regular use (see DOSAGE AND ADMINISTRATION).
Do not take extra doses or stop taking RHINOCORT AQUA without telling your healthcare provider.
Patients or their parents/guardians should inform their physician if they are allergic to any of the ingredients of RHINOCORT AQUA or any other intranasal corticosteroid.
Patients should inform their physician of other medications they are taking as RHINOCORT AQUA may not be suitable in some circumstances and the physician may wish to use a different medicine.
Long-term use of intranasal corticosteroids, including budesonide, may increase the risk of some eye problems (cataracts or glaucoma). Regular eye examinations should be considered.
An improvement in nasal symptoms may be noted in patients within 10 hours of first using RHINOCORT AQUA Nasal Spray. This time to onset is supported by an environmental exposure unit study in seasonal allergic rhinitis patients that demonstrated that RHINOCORT AQUA Nasal Spray led to a statistically significant improvement in nasal symptoms compared to placebo by 10 hours. Further support comes from a clinical study of patients with perennial allergic rhinitis which demonstrated a statistically significant improvement in nasal symptoms for both RHINOCORT AQUA Nasal Spray and for the active comparator (mometasone furoate) compared to placebo by 8 hours. Onset was also assessed in this study with peak nasal inspiratory flow rate and this endpoint failed to show efficacy for either active treatment. Although statistically significant improvements in nasal symptoms compared to placebo were noted within 8-10 hours in these studies, about one half to two thirds of the ultimate clinical improvement with RHINOCORT AQUA Nasal Spray occurs over the first 1-2 days, and maximum benefit may not be achieved until approximately 2 weeks after initiation of treatment. Initial assessment for response should be made during this time frame and periodically until the patient’s symptoms are stabilized.
The patient should take the medication as directed and should not exceed the prescribed dosage. The patient should contact the physician if symptoms do not improve after two weeks, or if the condition worsens. Patients who experience recurrent episodes of epistaxis (nosebleeds) or nasal septum discomfort while taking this medication should contact their physician. For proper use of this unit and to attain maximum improvement, the patient should read and follow the accompanying patient instructions carefully.
It is important to shake the bottle well before each use. The RHINOCORT AQUA Nasal Spray 32 mcg bottle has been filled with an excess to accommodate the priming activity. The bottle should be discarded after 120 sprays following initial priming, since the amount of budesonide delivered per spray thereafter may be substantially less than the labeled dose. Do not transfer any remaining suspension to another bottle.
Drug Interactions
The main route of metabolism of budesonide, as well as other corticosteroids, is via cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4). After oral administration of ketoconazole, a potent inhibitor of CYP3A4, the mean plasma concentration of orally administered budesonide increased by more than seven-fold. Concomitant administration of other known inhibitors of CYP3A4 (eg, itraconazole, clarithromycin, erythromycin, etc.) may inhibit the metabolism of, and increase the systemic exposure to, budesonide (see WARNINGS and PRECAUTIONS, General). Care should be exercised when budesonide is coadministered with long-term ketoconazole and other known CYP3A4 inhibitors.
Omeprazole, an inhibitor of CYP2C19, did not have effects on the pharmacokinetics of oral budesonide, while cimetidine, primarily an inhibitor of CYP1A2, caused a slight decrease in budesonide clearance and corresponding increase in its oral bioavailability.
Although there is no data with intranasal corticosteroids, an open-label non-randomized clinical study examined the immune responsiveness of varicella vaccine in 243 asthma patients 12 months to 8 years of age who were treated with budesonide inhalation suspension 0.25 mg to 1 mg daily (n=151) or non-corticosteroid asthma therapy (n=92) (ie, beta2-agonists, leukotriene receptor antagonists, cromones). The percentage of patients developing a seroprotective antibody titer ≥5.0 (gpELISA value) in response to the vaccination was similar in patients treated with budesonide inhalation suspension (85%) compared to patients treated with non-corticosteroids asthma therapy (90%). No patient treated with budesonide inhalation suspension developed chicken pox as a result of vaccination.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two-year study in Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of gliomas in the male rats receiving an oral dose of 50 mcg/kg (approximately twice the maximum recommended daily intranasal dose in adults and children on a mcg/m2 basis). No tumorigenicity was seen in male and female rats at respective oral doses up to 25 and 50 mcg/kg (approximately equal to and two times the maximum recommended daily intranasal dose in adults and children on a mcg/m2 basis, respectively). In two additional two-year studies in male Fischer and Sprague-Dawley rats, budesonide caused no gliomas at an oral dose of 50 mcg/kg (approximately twice the maximum recommended daily intranasal dose in adults and children on a mcg/m2 basis). However, in male Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of hepatocellular tumors at an oral dose of 50 mcg/kg (approximately twice the maximum recommended daily intranasal dose in adults and children on a mcg/m2 basis). The concurrent reference corticosteroids (prednisolone and triamcinolone acetonide) in these two studies showed similar findings.
In a 91-week study in mice, budesonide caused no treatment-related carcinogenicity at oral doses up to 200 mcg/kg (approximately 3 times the maximum recommended daily intranasal dose in adults and children on a mcg/m2 basis).
Budesonide was not mutagenic or clastogenic in six different test systems: Ames Salmonella/microsome plate test, mouse micronucleus test, mouse lymphoma test, chromosome aberration test in human lymphocytes, sex-linked recessive lethal test in Drosophila melanogaster, and DNA repair analysis in rat hematocyte culture.
In rats, budesonide caused a decrease in prenatal viability and viability of the pups at birth and during lactation, along with a decrease in maternal body-weight gain, at subcutaneous doses of 20 mcg/kg and above (less than the maximum recommended daily intranasal dose in adults on a mcg/m2 basis). No such effects were noted at 5 mcg/kg (less than the maximum recommended daily intranasal dose in adults on a mcg/m2 basis).
Pregnancy
Teratogenic Effects: Pregnancy category B
The impact of budesonide on human pregnancy outcomes has been evaluated through assessments of birth registries linked with maternal usage of inhaled budesonide (ie, PULMICORT TURBUHALER) and intranasally administered budesonide (ie, RHINOCORT AQUA Nasal Spray). The results from population-based prospective cohort epidemiological studies reviewing data from three Swedish registries covering approximately 99% of the pregnancies from 1995- 2001 (ie, Swedish Medical Birth Registry; Registry of Congenital Malformations; Child Cardiology Registry) indicate no increased risk for overall congenital malformations from the use of inhaled or intranasal budesonide during early pregnancy.
Congenital malformations were studied in 2,014 infants born to mothers reporting the use of inhaled budesonide for asthma in early pregnancy (usually 10-12 weeks after the last menstrual period), the period when most major organ malformations occur.1 The rate of overall congenital malformations was similar compared to the general population rate (3.8 % vs. 3.5%, respectively). The number of infants born with orofacial clefts and cardiac defects was similar to the expected number in the general population (4 children vs. 3.3 and 18 children vs. 17-18, respectively). In a follow-on study bringing the total number of infants to 2,534, the rate of overall congenital malformations among infants whose mothers were exposed to inhaled budesonide during early pregnancy was not different from the rate for all newborn babies during the same period (3.6%).2 A third study from the Swedish Medical Birth Registry of 2,968 pregnancies exposed to inhaled budesonide, the majority of which were first trimester exposures, reported gestational age, birth weight, birth length, stillbirths, and multiple births similar for exposed infants compared to nonexposed infants.3
Congenital malformations were studied in 2,113 infants born to mothers reporting the use of intranasal budesonide in early pregnancy. The rate of overall congenital malformations was similar compared to the general population rate (4.5% vs. 3.5%, respectively). The adjusted odds ratio (OR) was 1.06 (95% CI 0.86-1.31). The number of infants born with orofacial clefts was similar to the expected number in the general population (3 children vs. 3, respectively). The number of infants born with cardiac defects exceeded that expected in the general population (28 children vs. 17.8 respectively). The systemic exposure from intranasal budesonide is 6-fold less than from inhaled budesonide and an association of cardiac defects was not seen with higher exposures of budesonide.
As with other corticosteroids, budesonide was teratogenic and embryocidal in rabbits and rats. Budesonide produced fetal loss, decreased pup weights, and skeletal abnormalities at subcutaneous doses of 25 mcg/kg in rabbits and 500 mcg/kg in rats (approximately 2 and 16 times the maximum recommended daily intranasal dose in adults on a mcg/m2 basis). In another study in rats, no teratogenic or embryocidal effects were seen at inhalation doses up to 250 mcg/kg (approximately 8 times the maximum recommended daily intranasal dose in adults on a mcg/m2 basis).
Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans.
Despite the animal findings, it would appear that the possibility of fetal harm is remote if the drug is used during pregnancy. Nevertheless, because the studies in humans cannot rule out the possibility of harm, RHINOCORT AQUA should be used during pregnancy only if clearly needed.
- 1
- Kallen B, Rydhstroem H, Aberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstet Gynecol 1999;93:392-395.
- 2
- Ericson A, Kallen B. Use of drugs during pregnancy: unique Swedish registration method that can be improved. Swedish Medical Products Agency 1999;1:8-11
- 3
- Norjavaara E, Gerhardsson de Verdier M. Normal pregnancy outcomes in a population-based study including 2968 pregnant women exposed to budesonide. J Allergy Clin Immunol 2003;111:736-742.
Nonteratogenic Effects:
Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Such infants should be carefully observed.
Nursing Mothers
Budesonide, like other corticosteroids, is secreted in human milk. Data with budesonide delivered orally via dry powder inhaler to women with asthma indicates that the total daily oral dose of budesonide in breast milk to the infant is approximately 0.3% to 1% of the dose inhaled by the mother (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Special Populations, Nursing Mothers). No studies have been conducted in breastfeeding women specifically with RHINOCORT AQUA; however, the dose of budesonide available to the infant in breast milk, as a percentage of the maternal dose, would be expected to be similar. RHINOCORT AQUA should be used in nursing women only if clinically appropriate. Prescribers should weigh the known benefits of breastfeeding for the mother and infant against the potential risks of minimal budesonide exposure in the infant. Dosing considerations include prescription or titration to the lowest clinically effective dose and use of RHINOCORT AQUA immediately after breastfeeding to maximize the time interval between dosing and breastfeeding to minimize infant exposure. However, in general, RHINOCORT AQUA use should not delay or interfere with infant feeding.
Pediatric Use
Safety and effectiveness in pediatric patients below 6 years of age have not been established.
Controlled clinical studies have shown that intranasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA)-axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA-axis function. The long-term effects of this reduction in growth velocity associated with intranasal corticosteroids, including the impact on final adult height, are unknown. The potential for "catch-up" growth following discontinuation of treatment with intranasal corticosteroids has not been adequately studied.
The growth of pediatric patients receiving intranasal corticosteroids, including RHINOCORT AQUA Nasal Spray, should be monitored routinely (eg, via stadiometry). The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the risks and benefits associated with alternative therapies. To minimize the systemic effects of intranasal corticosteroids, including RHINOCORT AQUA Nasal Spray, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
A one-year placebo-controlled clinical growth study was conducted in 229 pediatric patients (ages 4 through 8 years of age) to assess the effect of RHINOCORT AQUA (single-daily dose of 64 mcg, the recommended starting dose for children ages 6 years and above) on growth velocity. From a population of 141 patients receiving RHINOCORT AQUA Nasal Spray and 67 receiving placebo, the point estimate for growth velocity with RHINOCORT AQUA Nasal Spray was 0.25 cm/year lower than that noted with placebo (95% confidence interval ranging from 0.59 cm/year lower than placebo to 0.08 cm/year higher than placebo).
In a study of asthmatic children 5-12 years of age, those treated with budesonide administered via a dry powder inhaler 200 mcg twice daily (n=311) had a 1.1-centimeter (0.433 inch) reduction in growth compared with those receiving placebo (n=418) at the end of one year; the difference between these two treatment groups did not increase further over three years of additional treatment. By the end of four years, children treated with budesonide dry powder inhaler and children treated with placebo had similar growth velocities. Conclusions drawn from this study may be confounded by the unequal use of corticosteroids in the treatment groups and inclusion of data from patients attaining puberty during the course of the study.
The systemic effects of inhaled corticosteroids are related to the systemic exposure to such drugs. Pharmacokinetic studies have demonstrated that in both adults and children, systemic exposure to budesonide at the highest recommended doses of RHINOCORT AQUA would be expected to be no greater than exposure at the lowest recommended doses via a dry powder inhaler. Therefore, the systemic effects (HPA axis and growth) of budesonide delivered from RHINOCORT AQUA would be expected to be no greater than what is reported for inhaled budesonide when administered via the dry powder inhaler.
The potential for RHINOCORT AQUA to cause growth suppression in susceptible patients or when given at doses above 64 mcg daily cannot be ruled out. The recommended dosage range in patients 6 to 11 years of age is 64 to 128 mcg per day (see DOSAGE AND ADMINISTRATION).
Geriatric Use
Of the 2,461 patients in clinical studies of RHINOCORT AQUA Nasal Spray, 5% were 60 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, except for an adverse event reporting frequency of epistaxis that increased with age. Further, other reported clinical experience has not identified any other differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
ADVERSE REACTIONS
The incidence of common adverse reactions is based upon two U.S. and five non-U.S. controlled clinical trials in 1,526 patients [110 females and 239 males less than 18 years of age, and 635 females and 542 males 18 years of age and older] treated with RHINOCORT AQUA Nasal Spray at doses up to 400 mcg once daily for 3-6 weeks. The table below describes adverse events occurring at an incidence of 2% or greater and more common among RHINOCORT AQUA Nasal Spray-treated patients than in placebo-treated patients in controlled clinical trials. The overall incidence of adverse events was similar between RHINOCORT AQUA and placebo.
|
Adverse Event |
RHINOCORT AQUA |
Placebo Vehicle |
|
Epistaxis |
8% |
5% |
|
Pharyngitis |
4% |
3% |
|
Bronchospasm |
2% |
1% |
|
Coughing |
2% |
<1% |
|
Nasal Irritation |
2% |
<1% |
A similar adverse event profile was observed in the subgroup of pediatric patients 6 to 12 years of age.
Two to three percent (2-3%) of patients in clinical trials discontinued because of adverse events. Systemic corticosteroid side effects were not reported during controlled clinical studies with RHINOCORT AQUA Nasal Spray.
If recommended doses are exceeded, however, or if individuals are particularly sensitive, symptoms of hypercorticism, ie, Cushing’s Syndrome, and adrenal suppression could occur.
Adverse events reported from post-marketing experience include: immediate and delayed hypersensitivity reactions (including anaphylactic reaction, urticaria, rash, dermatitis, angioedema and pruritus), glaucoma, increased intraocular pressure, cataracts, nasal septum perforation, pharynx disorders (throat irritation, throat pain, swollen throat, burning throat, and itchy throat), anosmia, and palpitations.
Cases of growth suppression have been reported for intranasal corticosteroids including RHINOCORT AQUA Nasal Spray (see PRECAUTIONS, Pediatric Use).
OVERDOSAGE
Acute overdosage with this dosage form is unlikely since one 120 spray bottle of RHINOCORT AQUA Nasal Spray 32 mcg only contains approximately 5.4 mg of budesonide. Chronic overdosage may result in signs/symptoms of hypercorticism (see WARNINGS and PRECAUTIONS).
DOSAGE AND ADMINISTRATION
The recommended starting dose for adults and children 6 years of age and older is 64 mcg per day administered as one spray per nostril of RHINOCORT AQUA Nasal Spray 32 mcg once daily. The maximum recommended dose for adults (12 years of age and older) is 256 mcg per day administered as four sprays per nostril once daily of RHINOCORT AQUA Nasal Spray 32 mcg and the maximum recommended dose for pediatric patients (<12 years of age) is 128 mcg per day administered as two sprays per nostril once daily of RHINOCORT AQUA Nasal Spray 32 mcg (see HOW SUPPLIED).
Prior to initial use, the container must be shaken gently and the pump must be primed by actuating eight times. If used daily, the pump does not need to be reprimed. If not used for two consecutive days, reprime with one spray or until a fine spray appears. If not used for more than 14 days, rinse the applicator and reprime with two sprays or until a fine spray appears.
Individualization of Dosage
It is always desirable to titrate an individual patient to the minimum effective dose to reduce the possibility of side effects. In adults and children 6 years of age and older, the recommended starting dose is 64 mcg daily administered as one spray per nostril of RHINOCORT AQUA Nasal Spray 32 mcg, once daily. Some patients who do not achieve symptom control at the recommended starting dose may benefit from an increased dose. The maximum daily dose is 256 mcg for adults and 128 mcg for pediatric patients (<12 years of age). When the maximum benefit has been achieved and symptoms have been controlled, reducing the dose may be effective in maintaining control of the allergic rhinitis symptoms in patients who were initially controlled on higher doses.
An improvement in nasal symptoms may be noted in patients within 10 hours of first using RHINOCORT AQUA Nasal Spray. This time to onset is supported by an environmental exposure unit study in seasonal allergic rhinitis patients that demonstrated that RHINOCORT AQUA Nasal Spray led to a statistically significant improvement in nasal symptoms compared to placebo by 10 hours. Further support comes from a clinical study of patients with perennial allergic rhinitis which demonstrated a statistically significant improvement in nasal symptoms for both RHINOCORT AQUA Nasal Spray and for the active comparator (mometasone furoate) compared to placebo by 8 hours. Onset was also assessed in this study with peak nasal inspiratory flow rate and this endpoint failed to show efficacy for either active treatment. Although statistically significant improvements in nasal symptoms compared to placebo were noted within 8-10 hours in these studies, about one half to two thirds of the ultimate clinical improvement with RHINOCORT AQUA Nasal Spray occurs over the first 1-2 days, and maximum benefit may not be achieved until approximately 2 weeks after initiation of treatment. Initial assessment for response should be made during this time frame and periodically until the patient’s symptoms are stabilized.
Directions for Use
Illustrated Patient's Instructions for Use accompany each package of RHINOCORT AQUA Nasal Spray 32 mcg.
HOW SUPPLIED
RHINOCORT AQUA Nasal Spray 32 mcg is available in an amber glass bottle with a metered-dose pump spray and a green protection cap. RHINOCORT AQUA Nasal Spray 32 mcg provides 120 metered sprays after initial priming; net fill weight 8.6 g. The RHINOCORT AQUA Nasal Spray 32 mcg bottle has been filled with an excess to accommodate the priming activity. The bottle should be discarded after 120 sprays following initial priming, since the amount of budesonide delivered per spray thereafter may be substantially less than the labeled dose. Each spray delivers 32 mcg of budesonide to the patient.
NDC 0186-1070-08
RHINOCORT AQUA Nasal Spray
32 mcg, 120 metered sprays; net fill weight 8.6 g
RHINOCORT AQUA Nasal Spray should be stored at controlled room temperature, 20 to 25°C (68 to 77°F) with the valve up. Do not freeze. Protect from light. Shake gently before use. Do not spray in eyes.
All trademarks are the property of the AstraZeneca group of companies
©AstraZeneca 2008
Distributed by:
AstraZeneca LP, Wilmington, DE 19850
Rev. 10/08 30516-XX
SUPPLEMENTAL PATIENT MATERIAL
Patient Instructions for Use
Instructions for using your RHINOCORT AQUA® (budesonide) Nasal Spray 32 mcg
IMPORTANT: Please read this leaflet carefully before you start to use RHINOCORT AQUA Nasal Spray. It provides a summary of information about your medicine. FOR FURTHER INFORMATION, ASK YOUR DOCTOR OR PHARMACIST.
What You Should Know about Rhinocort Aqua Nasal Spray
Your doctor has prescribed RHINOCORT AQUA Nasal Spray, a medicine that can help treat your nasal symptoms due to perennial or seasonal allergic rhinitis. “Rhinitis” means inflammation of the lining of the nose. RHINOCORT AQUA Nasal Spray contains budesonide, a synthetic corticosteroid. Corticosteroids are natural substances found in the body that help fight inflammation
What are the Symptoms of Perennial (Year-Round) or Seasonal Allergic Rhinitis?
Nasal symptoms of perennial (year-round) or seasonal allergic rhinitis include:
- runny nose
-
sneezing
-
stuffy nose
-
itching of the nose or throat
RHINOCORT AQUA helps to relieve these nasal symptoms.
Before using your Rhinocort Aqua Nasal Spray, tell your Doctor or Pharmacist if:
-
you are pregnant (or intending to become pregnant),
-
you are breast-feeding a baby,
-
you are allergic to budesonide, any of the other ingredients of RHINOCORT AQUA or any other nasal inhaled corticosteroid.
-
you have recently been around anyone with chicken pox or measles
-
you have liver problems
-
you have chronic or untreated infections
-
you recently had surgery or an injury to your nose
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal products. RHINOCORT AQUA and other medicines may affect each other, causing side effects.
Talk to your Doctor or Pharmacist if:
-
you have nose bleeds or pain or discomfort in your nose
-
your symptoms worsen
-
your symptoms do not begin to improve after 2 weeks of use
In some circumstances, this medicine may not be suitable, and your doctor may wish to give you a different medicine. Make sure that your doctor knows what other medicine you are taking.
What are the Possible Side Effects of Rhinocort Aqua?
The most common side effects reported by patients using RHINOCORT AQUA are:
-
nose bleeds
-
irritation to the nose
-
sore throat
-
breathing difficulties such as wheezing, chest tightening or coughing
In rare instances, severe allergic reaction and eye problems (including glaucoma and cataracts) have been reported.
These are not all of the possible side effects of RHINOCORT AQUA. For more information, ask your doctor or pharmacist.
Using Your Rhinocort Aqua Nasal Spray
-
Follow the instructions on the reverse side of this page. If you have any problems, tell your doctor or pharmacist.
-
It is important that you use RHINOCORT AQUA Nasal Spray as directed by your doctor. The pharmacy label will usually tell you what dose to take and how often. If it doesn’t or you are not sure how to use RHINOCORT AQUA Nasal Spray, ask your doctor or pharmacist.
Dosage
-
Use as directed by your doctor.
-
It is very important that you follow your doctor’s instructions as to how many sprays to take and how often to use your RHINOCORT AQUA Nasal Spray.
-
Do not take more doses or use RHINOCORT AQUA Nasal Spray more often than your doctor tells you.
-
It is very important that you use RHINOCORT AQUA NASAL SPRAY regularly. Do not stop treatment or reduce your dose, even if you are feeling better, unless told to do so by your doctor.
-
If you miss a dose, just take your regularly scheduled next dose when it is due. Do not double the dose.
-
If you also have itchy, watery eyes, you should tell your doctor. He or she can prescribe additional medication to treat these symptoms.
Storing Your Rhinocort Aqua Nasal Spray
-
Keep the green protective cap on RHINOCORT AQUA Nasal Spray when not in use. (Please see Prior to Use on reverse side).
-
Keep RHINOCORT AQUA Nasal Spray and all medications out of the reach of children.
-
Keep RHINOCORT AQUA Nasal Spray in a dry place at controlled room temperature, 68 to 77°F (20 to 25°C). Do not freeze. Protect from light.
-
Do not use RHINOCORT AQUA Nasal Spray after the labeled number of sprays have been used (does not include priming) or after the expiration date shown on the carton or bottle label.
What are the Ingredients of Rhinocort Aqua?
Active ingredient: budesonide 32 mcg
Inactive ingredients: Microcrystalline cellulose and carboxymethyl cellulose sodium, dextrose anhydrous, polysorbate 80, disodium edetate, potassium sorbate, and purified water are contained in this medium; hydrochloric acid is added to adjust the pH to a target of 4.5.
Further Information
This leaflet does not contain the complete information about your medicine. If you have any questions, or are not sure about something, you should ask your doctor or pharmacist.
You may want to read this leaflet again. Please do not throw it away until you have finished your medicine.
REMEMBER: This medicine has been prescribed for you by your doctor. Do not give this medicine to anyone else.
Use this product as directed, unless instructed to do otherwise by your doctor.
How To Use Your Rhinocort Aqua Nasal Spray
Read the complete instructions carefully and use only as directed.

Prior to Use: Before using RHINOCORT AQUA Nasal Spray, the bottle must be primed. To do this:
-
Pull to remove the green protective cap off the nasal spray unit.
-
Shake the bottle gently for a few seconds before each use.
-
Hold the bottle firmly, as shown in Figure A, with your index and middle finger on either side of the spray tip and your thumb underneath the bottle.
-
Actuate the pump by QUICKLY and FIRMLY pressing down on the white collar while supporting the base of the bottle with your thumb.
-
Prior to initial use, shake the bottle gently. The pump must be primed by actuating 8 times. If used daily the pump does not need to be reprimed. If not used for 2 consecutive days, reprime with one spray or until a fine spray appears. If not used for more than 14 days, rinse the applicator using the cleaning steps listed below. Reprime with two sprays or until a fine spray appears.
-
Do not spray in eyes.
NOTE: Your RHINOCORT AQUA Nasal Spray has been filled with an excess to accommodate the priming activity.
The correct amount of medication in each spray cannot be assured after the labeled number of sprays even though the container is not completely empty. Keep track of the number of sprays released from the container. Do not transfer any remaining RHINOCORT AQUA Nasal Spray to another bottle.
Instructions for Daily Use: Follow these instructions for daily use of RHINOCORT AQUA Nasal Spray:
-
Gently blow your nose to clear your nostrils, if necessary.
-
Shake the bottle gently for a few seconds and remove the green protective cap.
-
Hold the bottle firmly with your index and middle finger on either side of the spray tip and your thumb underneath the bottle (Figure A).
Figure A
-
Insert the spray tip into your nostril (the tip should not reach far into your nose). Close the other nostril with a finger and lean your head slightly forward so the spray will aim toward the back of your nose (Figure B).

Figure B
-
For each spray, actuate the pump by QUICKLY and FIRMLY pressing down on the white collar while supporting the base of the bottle with your thumb. Breathe gently inward through the nostril.
-
After spraying into your nostril, lean your head backward for a few seconds (Figure C).

Figure C
-
If a second spray is required in the same nostril, repeat steps 3 through 6.
-
Repeat steps 3 through 7 for your other nostril.
-
Avoid blowing your nose for 15 minutes after using RHINOCORT AQUA Nasal Spray.
-
Wipe the spray tip with a clean tissue (Figure D), and replace the green protective cap. Store the bottle in an upright position.
Figure D
Cleaning
Rinse the upper plastic parts regularly. To do this:
-
Remove the green protective cap and lift off the spray tip.
-
Wash only these plastic parts in warm water and rinse them in cold tap water.
-
Allow the plastic parts to air-dry completely before reassembling the nasal spray.
-
If the spray tip becomes blocked, it can be cleared by performing the above steps. Do not unblock the nasal applicator with a pin or other sharp object.
For additional information about RHINOCORT AQUA®, please call the AstraZeneca Information Center, Monday through Friday, 8 am – 7 pm ET, excluding holidays at 1-800-236-9933 or visit our website at rhinocortaqua.com.
All trademarks are the property of the AstraZeneca group of companies
©AstraZeneca 2002, 2008
Distributed by: AstraZeneca LP, Wilmington, DE 19850
30515-XX Rev. 11/08
PACKAGE LABEL PRINCIPAL DISPLAY PANEL

| RHINOCORT AQUA
budesonide spray, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA020746 | 10/05/2009 | |
| Labeler - AstraZeneca LP (176500158) |
| Registrant - AstraZeneca PLC (230790719) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| AstraZeneca AB | 353951254 | API MANUFACTURE, MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| AstraZeneca LP | 176500158 | MANUFACTURE, REPACK | |