GAZYVA- obinutuzumab injection, solution, concentrate
Genentech, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GAZYVA safely and effectively. See full prescribing information for GAZYVA.
GAZYVA™ (obinutuzumab) Injection, for intravenous infusion Initial U.S. Approval: 2013 WARNING: HEPATITIS B VIRUS REACTIVATION AND PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHYSee full prescribing information for complete boxed warning.INDICATIONS AND USAGEDOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONSNone. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions (incidence ≥ 10%) were: infusion reactions, neutropenia, thrombocytopenia, anemia, pyrexia, cough, and musculoskeletal disorder. (6) See 17 for PATIENT COUNSELING INFORMATION. Revised: 11/2013 |
FULL PRESCRIBING INFORMATION
WARNING: HEPATITIS B VIRUS REACTIVATION AND PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
- Hepatitis B Virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients receiving CD20-directed cytolytic antibodies, including GAZYVA. Screen all patients for HBV infection before treatment initiation. Monitor HBV positive patients during and after treatment with GAZYVA. Discontinue GAZYVA and concomitant medications in the event of HBV reactivation [see Warnings and Precautions (5.1)].
- Progressive Multifocal Leukoencephalopathy (PML) including fatal PML, can occur in patients receiving GAZYVA [see Warnings and Precautions (5.2)].
1 INDICATIONS AND USAGE
GAZYVA, in combination with chlorambucil, is indicated for the treatment of patients with previously untreated chronic lymphocytic leukemia (CLL) [see Clinical Studies (14.1)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage Regimen
- Premedicate before each infusion [see Dosage and Administration (2.2)].
- Administer only as an intravenous infusion through a dedicated line [see Dosage and Administration (2.5)].
- Do not administer as an intravenous push or bolus.
- Monitor blood counts at regular intervals.
- GAZYVA should only be administered by a healthcare professional with appropriate medical support to manage severe infusion reactions that can be fatal if they occur [see Warnings and Precautions (5.3)].
Recommended Dose:
Each dose of GAZYVA is 1000 mg, administered intravenously, with the exception of the first infusions in cycle 1, which are administered on day 1 (100 mg) and day 2 (900 mg).
| Day of treatment cycle | Dose of GAZYVA | Rate of infusion (in the absence of infusion reactions/hypersensitivity during previous infusions) | |
|---|---|---|---|
| Cycle 1 | Day 1 | 100 mg | Administer at 25 mg/hr over 4 hours. Do not increase the infusion rate. |
| Day 2 | 900 mg | Administer at 50 mg/hr. The rate of the infusion can be escalated in increments of 50 mg/hr every 30 minutes to a maximum rate of 400 mg/hr. | |
| Day 8 | 1000 mg | Infusions can be started at a rate of 100 mg/hr and increased by 100 mg/hr increments every 30 minutes to a maximum of 400 mg/hr. | |
| Day 15 | 1000 mg | ||
| Cycles 2–6 | Day 1 | 1000 mg | |
If a planned dose of GAZYVA is missed, administer the missed dose as soon as possible and adjust dosing schedule accordingly. If appropriate, patients who do not complete the Day 1 Cycle 1 dose may proceed to the Day 2 Cycle 1 dose.
If a patient experiences an infusion reaction of any grade during infusion, adjust the infusion as follows [see Warnings and Precautions (5.3)]:
- Grade 4 (life threatening): Stop infusion immediately and permanently discontinue GAZYVA therapy.
- Grade 3 (severe): Interrupt infusion and manage symptoms. Upon resolution of symptoms, consider restarting GAZYVA infusion at no more than half the previous rate (the rate being used at the time that the infusion reaction occurred) and, if patient does not experience any further infusion reaction symptoms, infusion rate escalation may resume at the increments and intervals as appropriate for the treatment cycle dose. Permanently discontinue treatment if patients experience a Grade 3 infusion related symptom at re-challenge.
- Grade 1–2 (mild to moderate): Reduce infusion rate or interrupt infusion and treat symptoms. Upon resolution of symptoms, continue or resume infusion and, if patient does not experience any further infusion reaction symptoms, infusion rate escalation may resume at the increments and intervals as appropriate for the treatment cycle dose.
2.2 Recommended Premedication
Premedication is recommended to reduce the risk of infusion reactions as outlined in Table 2 [see Warnings and Precautions (5.3)].
Hypotension may occur during GAZYVA intravenous infusions. Consider withholding antihypertensive treatments for 12 hours prior to and throughout each GAZYVA infusion and for the first hour after administration [see Warnings and Precautions (5.3)].
For patients with high tumor burden and/or high circulating absolute lymphocyte counts (greater than 25 × 109/L), premedicate with anti-hyperuricemics (e.g., allopurinol) beginning 12–24 hours prior to start of therapy and ensure adequate hydration for prophylaxis of tumor lysis syndrome [see Warnings and Precautions (5.4)].
| Day of Treatment Cycle | Patients requiring premedication | Premedication | Administration |
|---|---|---|---|
|
|||
| Cycle 1: Day 1, Day 2 | All patients | Intravenous glucocorticoid: 20 mg dexamethasone or 80 mg methylprednisolone * | Completed at least 1 hour prior to GAZYVA infusion. |
| 650–1000 mg Acetaminophen | At least 30 minutes before GAZYVA infusion. | ||
| Anti-histamine (e.g., diphenhydramine 50 mg) | |||
| Cycle 1: Day 8, Day 15 Cycles 2–6: Day 1 | All patients | 650–1000 mg Acetaminophen | At least 30 minutes before GAZYVA infusion. |
| Patients with an IRR (≥ Grade 1) with the previous infusion | Anti-histamine (e.g., diphenhydramine 50 mg) | At least 30 minutes before GAZYVA infusion. | |
| Patients with a Grade 3 IRR with the previous infusion OR with a lymphocyte count > 25 × 109/L prior to next treatment | Intravenous glucocorticoid: 20 mg dexamethasone or 80 mg methylprednisolone* | Completed at least 1 hour prior to GAZYVA infusion. | |
2.3 Premedication for anti-microbial prophylaxis
Patients with neutropenia are strongly recommended to receive antimicrobial prophylaxis throughout the treatment period. Antiviral and antifungal prophylaxis should be considered.
2.4 Treatment Interruption for Toxicity
Consider treatment interruption, if patients experience an infection, Grade 3 or 4 cytopenia, or a ≥ Grade 2 non-hematologic toxicity.
2.5 Preparation and Administration
Preparation
Prepare the solution for infusion, using aseptic technique, as follows:
- Inspect visually for any particulate matter and discoloration prior to administration.
- Dilute into a 0.9% sodium chloride PVC or non-PVC polyolefin infusion bag. Do not use other diluents such as dextrose (5%).
- Preparation of solution for infusion on Day 1 (100 mg) and Day 2 (900 mg) of Cycle 1:
- Withdraw 40 mL of GAZYVA solution from the vial.
- Dilute 4 mL (100 mg) of GAZYVA into a 100 mL 0.9% sodium chloride infusion bag for immediate administration.
- Dilute the remaining 36 mL (900 mg) into a 250 mL 0.9% sodium chloride infusion bag at the same time for use on Day 2 and store at 2°C to 8°C (36°F to 46°F) for up to 24 hours. After allowing the diluted bag to come to room temperature, use immediately.
- Clearly label each infusion bag.
- Preparation of solution for infusion on Day 8 and 15 of Cycle 1 and Day 1 Cycles 2–6:
- Withdraw 40 mL of GAZYVA solution from the vial.
- Dilute 40 mL (1000 mg) into a 250 mL 0.9% sodium chloride infusion bag.
- Mix diluted solution by gentle inversion. Do not shake or freeze.
- For microbiological stability, the diluted GAZYVA infusion solution should be used immediately. Dilute under appropriate aseptic conditions. If not used immediately, the solution may be stored in a refrigerator at 2°C to 8°C (36°F to 46°F) for up to 24 hours prior to use.
The product can be administered at a final concentration of 0.4 mg/mL to 4 mg/mL.
Administration
- Administer as an intravenous infusion only.
- Do not administer as an intravenous push or bolus.
- Do not mix GAZYVA with other drugs.
- No incompatibilities between GAZYVA and polyvinylchloride (PVC) or non-PVC polyolefin bags and administration sets have been observed [see How Supplied/Storage and Handling (16.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Hepatitis B Virus Reactivation
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure and death, can occur in patients treated with anti-CD20 antibodies such as GAZYVA. HBV reactivation has been reported in patients who are hepatitis B surface antigen (HBsAg) positive and also in patients who are HBsAg negative but are hepatitis B core antibody (anti-HBc) positive. Reactivation has also occurred in patients who appear to have resolved hepatitis B infection (i.e., HBsAg negative, anti-HBc positive, and hepatitis B surface antibody [anti-HBs] positive).
HBV reactivation is defined as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA level or detection of HBsAg in a person who was previously HBsAg negative and anti-HBc positive. Reactivation of HBV replication is often followed by hepatitis, i.e., increase in transaminase levels and, in severe cases, increase in bilirubin levels, liver failure, and death.
Screen all patients for HBV infection by measuring HBsAg and anti-HBc before initiating treatment with GAZYVA. For patients who show evidence of hepatitis B infection (HBsAg positive [regardless of antibody status] or HBsAg negative but anti-HBc positive), consult physicians with expertise in managing hepatitis B regarding monitoring and consideration for HBV antiviral therapy.
Monitor patients with evidence of current or prior HBV infection for clinical and laboratory signs of hepatitis or HBV reactivation during and for several months following treatment with GAZYVA. HBV reactivation has been reported for other CD20-directed cytolytic antibodies following completion of therapy.
In patients who develop reactivation of HBV while receiving GAZYVA, immediately discontinue GAZYVA and any concomitant chemotherapy, and institute appropriate treatment. Resumption of GAZYVA in patients whose HBV reactivation resolves should be discussed with physicians with expertise in managing hepatitis B. Insufficient data exist regarding the safety of resuming GAZYVA in patients who develop HBV reactivation.
5.2 Progressive Multifocal Leukoencephalopathy
JC virus infection resulting in progressive multifocal leukoencephalopathy (PML), which can be fatal, was observed in patients treated with GAZYVA. Consider the diagnosis of PML in any patient presenting with new onset or changes to pre-existing neurologic manifestations. Evaluation of PML includes, but is not limited to, consultation with a neurologist, brain MRI, and lumbar puncture. Discontinue GAZYVA therapy and consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML.
5.3 Infusion Reactions
GAZYVA can cause severe and life-threatening infusion reactions. Two-thirds of patients experienced a reaction to the first 1000 mg infused of GAZYVA. Infusion reactions can also occur with subsequent infusions. Symptoms may include hypotension, tachycardia, dyspnea, and respiratory symptoms (e.g., bronchospasm, larynx and throat irritation, wheezing, laryngeal edema). Other common symptoms include nausea, vomiting, diarrhea, hypertension, flushing, headache, pyrexia, and chills [see Adverse Reactions (6.1)].
Premedicate patients with acetaminophen, anti-histamine, and a glucocorticoid. Institute medical management (e.g., glucocorticoids, epinephrine, bronchodilators, and/or oxygen) for infusion reactions as needed. Closely monitor patients during the entire infusion. Infusion reactions within 24 hours of receiving GAZYVA have occurred [see Dosage and Administration (2)].
For patients with any Grade 4 infusion reactions, including but not limited to anaphylaxis, acute life-threatening respiratory symptoms, or other life-threatening infusion reaction: Stop the GAZYVA infusion. Permanently discontinue GAZYVA therapy.
For patients with Grade 1, 2, or 3 infusion reactions: Interrupt GAZYVA for Grade 3 reactions until resolution of symptoms. Interrupt or reduce the rate of the infusion for Grade 1 or 2 reactions and manage symptoms [see Dosage and Administration (2)].
For patients with pre-existing cardiac or pulmonary conditions, monitor more frequently throughout the infusion and the post-infusion period since they may be at greater risk of experiencing more severe reactions. Hypotension may occur as part of the GAZYVA infusion reaction. Consider withholding antihypertensive treatments for 12 hours prior to, during each GAZYVA infusion, and for the first hour after administration until blood pressure is stable. For patients at increased risk of hypertensive crisis, consider the benefits versus the risks of withholding their hypertensive medication as is suggested here.
5.4 Tumor Lysis Syndrome
Acute renal failure, hyperkalemia, hypocalcemia, hyperuricemia, and/or hyperphosphatemia from Tumor Lysis Syndrome (TLS) can occur within 12–24 hours after the first infusion. Patients with high tumor burden and/or high circulating lymphocyte count (> 25 × 109/L) are at greater risk for TLS and should receive appropriate tumor lysis prophylaxis with anti-hyperuricemics (e.g., allopurinol) and hydration beginning 12–24 hours prior to the infusion of GAZYVA [see Dosage and Administration (2.2)]. For treatment of TLS, correct electrolyte abnormalities, monitor renal function, and fluid balance, and administer supportive care, including dialysis as indicated.
5.5 Infections
Serious bacterial, fungal, and new or reactivated viral infections can occur during and following GAZYVA therapy. Do not administer GAZYVA to patients with an active infection. Patients with a history of recurring or chronic infections may be at increased risk of infection.
5.6 Neutropenia
GAZYVA in combination with chlorambucil caused Grade 3 or 4 neutropenia in 34% of patients in the trial. Patients with Grade 3 to 4 neutropenia should be monitored frequently with regular laboratory tests until resolution. Anticipate, evaluate, and treat any symptoms or signs of developing infection.
Neutropenia can also be of late onset (occurring more than 28 days after completion of treatment) and/or prolonged (lasting longer than 28 days).
Patients with neutropenia are strongly recommended to receive antimicrobial prophylaxis throughout the treatment period. Antiviral and antifungal prophylaxis should be considered.
5.7 Thrombocytopenia
GAZYVA in combination with chlorambucil caused Grade 3 or 4 thrombocytopenia in 12% of patients in the trial. In 5% of patients, GAZYVA caused an acute thrombocytopenia occurring within 24 hours after the GAZYVA infusion. In patients with Grade 3 or 4 thrombocytopenia, monitor platelet counts more frequently until resolution. Transfusion of blood products (i.e., platelet transfusion) may be necessary.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Hepatitis B reactivation [see Warnings and Precautions (5.1)]
- Progressive multifocal leukoencephalopathy [see Warnings and Precautions (5.2)]
- Infusion reactions [see Warnings and Precautions (5.3)]
- Tumor lysis syndrome [see Warnings and Precautions (5.4)]
- Infections [see Warnings and Precautions (5.5)]
- Neutropenia [see Warnings and Precautions (5.6)]
- Thrombocytopenia [see Warnings and Precautions (5.7)]
The most common adverse reactions (incidence ≥ 10%) were: infusion reactions, neutropenia, thrombocytopenia, anemia, pyrexia, cough, and musculoskeletal disorders.
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in Tables 3 and 4 below are based on a total of 356 previously untreated patients with CLL during treatment with GAZYVA in combination with chlorambucil or with chlorambucil alone. Patients received three 1000 mg doses of GAZYVA on the first cycle and a single dose of 1000 mg once every 28 days for 5 additional cycles in combination with chlorambucil (6 cycles of 28 days each in total). In the last 45 patients enrolled, the first dose of GAZYVA was split between day 1 (100 mg) and day 2 (900 mg) [see Dosage and Administration (2.1)]. In total, 81% of patients received all 6 cycles (of 28 days each) of GAZYVA based therapy.
| Adverse Reactions (MedDRA*) System Organ Class | GAZYVA + Chlorambucil n = 240 | Chlorambucil n = 116 |
||
|---|---|---|---|---|
| All Grades % | Grades 3–4† % | All Grades % | Grades 3–4† % | |
|
||||
| Injury, Poisoning and Procedural Complications | ||||
| Infusion related reactions | 69 | 21 | 0 | 0 |
| Blood and lymphatic system disorders‡ | ||||
| Neutropenia | 40 | 34 | 18 | 16 |
| Thrombocytopenia | 15 | 11 | 7 | 3 |
| Anemia | 12 | 4 | 10 | 5 |
| Leukopenia | 7 | 5 | 0 | 0 |
| General disorders and administration site conditions | ||||
| Pyrexia | 10 | < 1 | 7 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||||
| Cough | 10 | 0 | 7 | < 1 |
| Investigations | GAZYVA + Chlorambucil n = 240 | Chlorambucil n = 116 |
||
|---|---|---|---|---|
| All Grades % | Grades 3–4 % | All Grades % | Grades 3–4 % | |
| Hematology | ||||
| Neutropenia | 77 | 46 | 53 | 27 |
| Lymphopenia | 80 | 40 | 9 | 2 |
| Leukopenia | 84 | 36 | 12 | < 1 |
| Thrombocytopenia | 47 | 14 | 50 | 11 |
| Chemistry | ||||
| Hypocalcemia | 32 | 3 | 29 | < 1 |
| Hyperkalemia | 31 | 5 | 17 | 2 |
| Hyponatremia | 29 | 8 | 11 | 2 |
| AST (SGOT increased) | 28 | < 1 | 12 | 0 |
| Creatinine increased | 28 | < 1 | 18 | < 1 |
| ALT (SGPT increased) | 25 | < 1 | 14 | 0 |
| Hypoalbuminemia | 22 | < 1 | 14 | < 1 |
| Alkaline Phosphatase increased | 16 | 0 | 11 | 0 |
| Hypokalemia | 13 | 1 | 4 | < 1 |
Infusion reactions: The incidence of infusion reactions was 69% with the first infusion of GAZYVA. The incidence of Grade 3 or 4 infusion reactions was 21% with 8% of patients discontinuing therapy. The incidence of reactions with subsequent infusions was 3% with the second 1000 mg and < 1% thereafter. No Grade 3 or 4 infusion reactions were reported beyond the first 1000 mg infused.
Of the first 53 patients receiving GAZYVA on the trial, 47 (89%) experienced an infusion reaction. After this experience, study protocol modifications were made to require pre-medication with a corticosteroid, anti-histamine, and acetaminophen. The first dose was also divided into two infusions (100 mg on day 1 and 900 mg on day 2). For the 45 patients for whom these mitigation measures were implemented, 21 patients (47%) experienced a reaction with the first 1000 mg and < 2% thereafter [see Dosage and Administration (2)].
Neutropenia: The incidence of neutropenia reported as an adverse reaction was 40% in the GAZYVA treated arm and 18% in the chlorambucil alone arm with the incidence of serious adverse events being 1% and 0%, respectively (Table 3). Cases of late onset neutropenia (occurring 28 days after completion of treatment or later) were 16% in the GAZYVA treated arm and 12% in the chlorambucil alone arm.
Infection: The incidence of infections was similar between arms. Thirty-eight percent of patients in the GAZYVA treated arm experienced an infection, 9% were Grade 3–4 and none were fatal.
Thrombocytopenia: The incidence of thrombocytopenia reported as an adverse reaction was 15% in the GAZYVA treated arm and 7% in the chlorambucil alone arm (Table 3). Five percent of patients in the GAZYVA treated arm experienced acute thrombocytopenia (occurring within 24 hours after the GAZYVA infusion).
6.2 Immunogenicity
Serum samples from patients with previously untreated CLL were tested during and after treatment for antibodies to GAZYVA. Approximately 13% (9/70) of GAZYVA treated patients tested positive for anti-GAZYVA antibodies at one or more time points during the 12 month follow-up period. Neutralizing activity of anti-GAZYVA antibodies has not been assessed.
Immunogenicity data are highly dependent on the sensitivity and specificity of the test methods used. Additionally, the observed incidence of a positive result in a test method may be influenced by several factors, including sample handling, timing of sample collection, drug interference, concomitant medication and the underlying disease. Therefore, comparison of the incidence of antibodies to GAZYVA with the incidence of antibodies to other products may be misleading. Clinical significance of anti-GAZYVA antibodies is not known.
6.3 Additional Clinical Trial Experience
Progressive multifocal leukoencephalopathy: PML has been reported with GAZYVA [see Warnings and Precautions (5.2)].
Worsening of pre-existing cardiac conditions: Fatal cardiac events have been reported in patients treated with GAZYVA.
Hepatitis B reactivation: Hepatitis B virus reactivation has been reported with GAZYVA [see Warnings and Precautions (5.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Risk Summary
There are no adequate and well-controlled studies of GAZYVA in pregnant women. Women of childbearing potential should use effective contraception while receiving GAZYVA and for 12 months following treatment. GAZYVA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
In a pre- and post-natal development study, pregnant cynomolgus monkeys received weekly intravenous doses of 25 or 50 mg/kg obinutuzumab from day 20 of pregnancy until parturition. There were no teratogenic effects in animals. The high dose results in an exposure (AUC) that is 2.4 times the exposure in patients with CLL at the recommended label dose. When first measured on Day 28 postpartum, obinutuzumab was detected in offspring and B cells were completely depleted. The B-cell counts returned to normal levels, and immunologic function was restored within 6 months after birth.
8.3 Nursing Mothers
It is not known whether obinutuzumab is excreted in human milk. However, obinutuzumab is excreted in the milk of lactating cynomolgus monkeys and human IgG is known to be excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from GAZYVA, a decision should be made whether to discontinue nursing, or discontinue drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of GAZYVA in pediatric patients has not been established.
8.5 Geriatric Use
Of 240 previously untreated CLL patients who received GAZYVA in combination with chlorambucil, 196 patients (82%) were ≥ 65 years of age and 109 patients (45%) were ≥ 75 years of age. The median age was 74 years. Of the 109 patients ≥ 75 years of age, 49 (45%) experienced serious adverse events and 5 (5%) experienced adverse events leading to death. For 131 patients < 75 years of age, 39 (30%) experienced a serious adverse event and 3 (2%) an adverse event leading to death. Similar rates were observed in the comparator arm. No significant differences in efficacy were observed between patients ≥ 75 years of age and those < 75 years of age [see Clinical Studies (14.1)].
8.6 Renal Impairment
Based on population pharmacokinetic analysis, a baseline creatinine clearance (CLcr) > 30 mL/min does not affect the pharmacokinetics of GAZYVA. GAZYVA has not been studied in patients with a baseline CLcr < 30 mL/min [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
There has been no experience with overdose in human clinical trials. Doses ranging from 50 mg up to and including 2000 mg per infusion have been administered in clinical trials. For patients who experience overdose, treatment should consist of immediate interruption or reduction of GAZYVA and supportive therapy.
11 DESCRIPTION
GAZYVA (obinutuzumab) is a humanized anti-CD20 monoclonal antibody of the IgG1 subclass. It recognizes a specific epitope of the CD20 molecule found on B-cells. The molecular mass of the antibody is approximately 150 kDa.
GAZYVA is produced by mammalian cell (CHO) suspension culture. GAZYVA is a sterile, clear, colorless to slightly brown, preservative free liquid concentrate for intravenous administration. GAZYVA is supplied at a concentration of 25 mg/mL in 1000 mg single use vials. The product is formulated in 20 mM L-histidine/L-histidine hydrochloride, 240 mM trehalose, 0.02% poloxamer 188. The pH is 6.0.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Obinutuzumab is a monoclonal antibody that targets the CD20 antigen expressed on the surface of pre B- and mature B-lymphocytes. Upon binding to CD20, obinutuzumab mediates B-cell lysis through (1) engagement of immune effector cells, (2) by directly activating intracellular death signaling pathways and/or (3) activation of the complement cascade. The immune effector cell mechanisms include antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis.
12.2 Pharmacodynamics
In clinical trials in patients with CLL, GAZYVA caused CD19 B-cell depletion (defined as CD19 B-cell counts < 0.07 × 109/L). Initial CD19 B-cell recovery was observed in some patients approximately 9 months after the last GAZYVA dose. At 18 months of follow up, some patients remain B-cell depleted.
Although the depletion of B-cells in the peripheral blood is a measurable pharmacodynamic effect, it is not directly correlated with the depletion of B-cells in solid organs or in malignant deposits. B-cell depletion has not been shown to be directly correlated to clinical response.
12.3 Pharmacokinetics
Based on a population pharmacokinetic (pop-PK) analysis, the geometric mean (CV%) volume of distribution of obinutuzumab at steady state is approximately 3.8 (23) L.
The elimination of obinutuzumab is comprised of a linear clearance pathway and a time-dependent non-linear clearance pathway. As GAZYVA treatment progresses, the impact of the time-dependent pathway diminishes in a manner suggesting target mediated drug disposition (TMDD). Based on a pop-PK analysis, the geometric mean (CV%) terminal obinutuzumab clearance and half-life are approximately 0.09 (46%) L/day and 28.4 (43%) days, respectively.
Specific Populations:
Body Weight: Volume of distribution and steady state clearance both increased with body weight, however, the expected change in exposure does not warrant a dose modification.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with obinutuzumab.
No specific studies have been conducted to evaluate potential effects on fertility; however, no adverse effects on male or female reproductive organs were observed in the 26-week repeat-dose toxicity study in cynomolgus monkeys.
14 CLINICAL STUDIES
14.1 Chronic Lymphocytic Leukemia
GAZYVA was evaluated in a three arm, open-label, active control, randomized, multicenter trial (Study 1) in patients with previously untreated CD20+ chronic lymphocytic leukemia requiring treatment and had coexisting medical conditions or reduced renal function as measured by creatinine clearance (CrCl) < 70 mL/min. Patients with CrCl < 30 mL/min, active infections, positive hepatitis B (HBsAg or anti-HBc positive, patients positive for anti-HBc could be included if hepatitis B viral DNA was not detectable) and hepatitis C serology, or immunization with live virus vaccine within 28 days prior to randomization were excluded from the trial. Patients were treated with chlorambucil control (Arm 1), GAZYVA in combination with chlorambucil (Arm 2) or rituximab in combination with chlorambucil (Arm 3). The safety and efficacy of GAZYVA was evaluated in a comparison of Arm 1 vs. Arm 2 in 356 patients. Data comparing Arm 2 vs. Arm 3 are not available at this time.
The majority of patients received 1000 mg of GAZYVA on days 1, 8, and 15 of the first cycle, followed by treatment on the first day of 5 subsequent cycles (total of 6 cycles, 28 days each). The first dose of GAZYVA was divided between day 1 (100 mg) and day 2 (900 mg) [see Dosage and Administration (2.1)], which was implemented in 45 patients. Chlorambucil was given orally at 0.5 mg/kg on day 1 and day 15 of all treatment cycles (1 to 6).
In Study 1, the median age was 73 years, 60% were male, and 95% were Caucasian. Sixty-eight percent had a CrCl < 70 mL/min and 76% had multiple coexisting medical conditions. Twenty-two percent of patients were Binet stage A, 42% were stage B, and 36% were stage C. The median estimated CrCl was 61 mL/min. Eighty-one percent of patients treated with GAZYVA in combination with chlorambucil received all 6 cycles compared to 67% of patients in the chlorambucil alone arm.
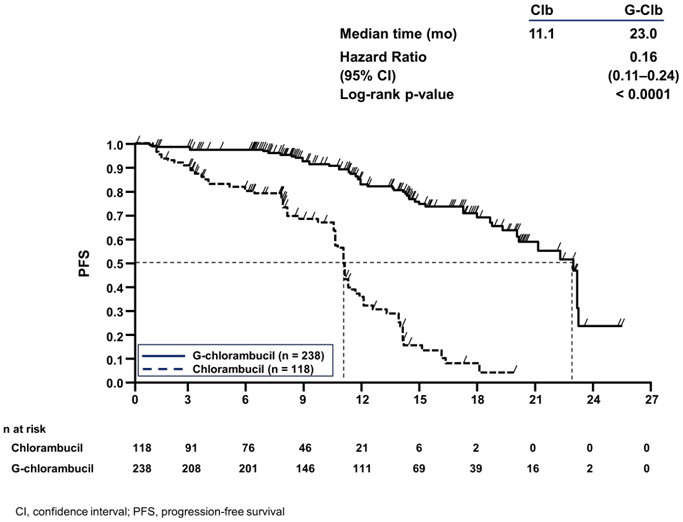
The median progression free survival (PFS) in the GAZYVA in combination with chlorambucil arm was 23.0 months and 11.1 months in the chlorambucil alone arm (median observation time 14.2 months) as assessed by independent review and is consistent with investigator assessed PFS. Efficacy results are shown in Table 5 and the Kaplan-Meier curve for PFS is shown in Figure 1.
| Endpoint | GAZYVA + Chlorambucil | Chlorambucil |
|---|---|---|
| Median Progression-Free Survival* | 23.0 months | 11.1 months |
| (HR 0.16 [0.11; 0.24], p-value < 0.0001 stratified log-rank test) | ||
| Overall Response Rate† | 75.9% | 32.1% |
| Complete Response | 27.8% | 0.9% |
| Median Duration of Response | 15.2 months | 3.5 months |
| Figure 1 |
| Kaplan-Meier Curve of Progression-Free Survival in Patients with CLL in Study 1 |
 |
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied/Storage
GAZYVA 1000 mg/40 mL (25 mg/mL) single-use vials containing preservative-free solution (NDC 50242-070-01) are stable at 2°C to 8°C (36°F to 46°F). Do not use beyond expiration date stamped on carton. GAZYVA vials should be protected from light. DO NOT FREEZE. DO NOT SHAKE.
For the diluted product, chemical and physical stability have been demonstrated in 0.9% NaCl at concentrations of 0.4 mg/mL to 20 mg/mL for 24 hours at 2°C to 8°C (36°F to 46°F) followed by 48 hours (including infusion time) at room temperature (≤ 30°C/86°F). GAZYVA does not contain antimicrobial preservatives. Therefore care must be taken to ensure that the solution for infusion is not microbiologically compromised during preparation. The solution for infusion should be used immediately. If not used immediately, the prepared solution may be stored up to 24 hours at 2–8°C. No incompatibilities between GAZYVA and polyvinyl chloride or polyolefin infusion materials have been observed in concentration ranges from 0.4 mg/mL to 20.0 mg/mL after dilution of GAZYVA with 0.9% sodium chloride.
17 PATIENT COUNSELING INFORMATION
Advise patients to seek immediate medical attention for any of the following:
- Signs and symptoms of infusion reactions including dizziness, nausea, chills, fever, vomiting, diarrhea, breathing problems, or chest pain [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
- Symptoms of tumor lysis syndrome such as nausea, vomiting, diarrhea, and lethargy [see Warnings and Precautions (5.4) and Adverse Reactions (6.1)].
- Signs of infections including fever and cough [see Warnings and Precautions (5.5) and Adverse Reactions (6.1)].
- Symptoms of hepatitis including worsening fatigue or yellow discoloration of skin or eyes [see Warnings and Precautions (5.1)].
- New or changes in neurological symptoms such as confusion, dizziness or loss of balance, difficulty talking or walking, or vision problems [see Warnings and Precautions (5.2)].
Advise patients of the need for:
- Periodic monitoring of blood counts [see Warnings and Precautions (5.6 and 5.7) and Adverse Reactions (6.1)].
- Avoid vaccinations with live viral vaccines [see Warnings and Precautions (5.8)].
- Patients with a history of hepatitis B infection (based on the blood test) should be monitored and sometimes treated for their hepatitis [see Warnings and Precautions (5.1)].
GAZYVA™ [obinutuzumab]
Manufactured by:
Genentech, Inc.
A Member of the Roche Group
South San Francisco, CA 94080-4990
U.S. License No: 1048
GAZYVA is a trademark of Genentech, Inc.
© 2013 Genentech, Inc.
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
| GAZYVA
obinutuzumab injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genentech, Inc. (080129000) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Diagnostics GmbH | 315028860 | MANUFACTURE(50242-070), ANALYSIS(50242-070) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| F. Hoffmann-La Roche Ltd | 485244961 | LABEL(50242-070), PACK(50242-070) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Diagnostics GmbH | 323105205 | API MANUFACTURE(50242-070) | |
