BEYONDCLEAR- salicylic acid and benzoyl peroxide
Proskin LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
When using this product
For Pure
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time unless directed by a doctor
- Avoid contact with the eyes. If contact occurs, immediately flush with water.
For Clear
- skin irritation and dryness is more likely to occur if use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor
- avoid contact with the eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently, in smaller amount or in a lower concentration.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
For Pure
Use in the morning and evening. Put a nickel- sized amount in hand
- Cover the entire area of concern with a thin layer Gently massage on to wet skin. Rinse and pat dry
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
For Clear
Use in the morning and evening after cleansing
- Clean the skin thoroughly before applying this product
- Cover the entire area of concern with a thin layer two times daily. Hold can upright. Dispense a dime-sized amount of product in your hand. Gently massage into skin. No need to shake container. Store upright
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
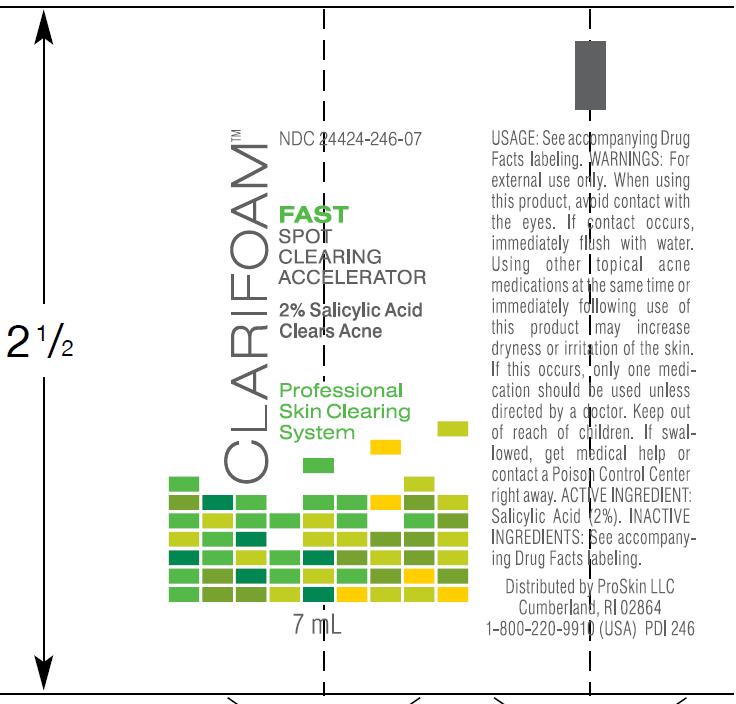
For FAST
- Clean the skin thoroughly before applying this product. Use as needed and on-the-go
- Apply a thin layer directly on the breakout area. Start with one application daily, and increase to two if needed. Do not rinse off
- If bothersome dryness occurs, reduce usage
- Sun protection is recommended by dermatologists as an essential part of any healthy skin care regimen.
Inactive Ingredients
For Pure
Purified Water, Glycerin, PEG-120 Methyl Glucose Dioleate, Cocamidopropyl Betaine, Sodium Cocoamphoacetate, PEG-200 Hydrogenated Glyceryl Palmate, Glucoside, Disodium Cocoyl Glutamate, Xylitylglucoside, Sodium Cocoyl Glutamate, Sodium Lauryl Glucose Carboxylate, Acer Saccharum (Sugar Maple) Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Saccharum Officinarum (Sugar Cane) Extract, Vaccinium Myrtillus Fruit/Leaf Extract, Anhydroxylitol, Lappa Root Extract, Citrus Limon (Lemon) Extract, Glycolic Acid, Hydroxyphenyl Propamidobenzoic Acid, Mandelic Acid, Sisymbrium Officinale Extract, Xylitol, Zinc PCA, PEG-7 Glyceryl Cocoate, Pentylene Glycol, Benzyl Alcohol, Butylene Glycol, Citric Acid, Sclerotium Gum, Fragrance, Polysorbate 20.
For Clear
Water, Hydrofluorocarbon 227ea, Glycerin, Pentylene Glycol, Emulsifying Wax, Dimethicone, Chamomilla Recutita (Matricaria) Flower Extract, Symphytum Officinale Extract, Phenoxyetha- nol, Steareth-10, C12-15 Alkyl Benzoate, Sodium Citrate, Cetearyl Alcohol, Fragrance, Disodium EDTA, BHT, Citric Acid, Aloe Barbadensis Leaf Juice.
For FAST
Purified Water, SD Alcohol 40-B, Butylene Glycol, Lactobacillus/Wasabia Japonica Root Ferment Extract, Glycerin, Polyacrylate Crosspolymer- 6, Allantoin, Bisabolol, Epilobium Fleischeri Extract, Juglans Nigra (Black Walnut) Shell Extract, Spiraea Ulmaria Extract, Hydroxyphenyl Propamidobenzoic Acid, Disodium EDTA, Pentylene Glycol, Ethylhexylglycerin, Phenoxyethanol.
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - PURE PURIFYING CLEANSER Label
NDC 24424-248-96
CLARIFOAMTM
1 PURE
PURIFYING
CLEANSER
2% Salicylic Acid
Clears Acne
Professional
Skin Clearing
System
125 mL

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - CLEAR CLARIFYING TREATMENT Label
NDC 24424-328-50
CLARIFOAMTM
2 CLEAR
CLARIFYING
TREATMENT
4.25% Benzoyl Peroxide
Clears Acne
Professional
Skin Clearing
System
50g

| BEYONDCLEAR
salicylic acid and benzoyl peroxide kit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Proskin LLC (969956668) |
| Registrant - Precision Dermatology, Inc. (056267369) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Precision Dermatology, Inc. | 056267369 | MANUFACTURE(24424-351) | |