HALAVEN- eribulin mesylate injection
Eisai Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HALAVEN™® safely and effectively. See full prescribing information for HALAVEN.
HALAVEN® (eribulin mesylate) Injection For intravenous administration. Initial U.S. Approval: 2010 INDICATIONS AND USAGEHALAVEN is a microtubule inhibitor indicated for the treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSCONTRAINDICATIONSNone (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions (incidence ≥25%) were neutropenia, anemia, asthenia/fatigue, alopecia, peripheral neuropathy, nausea, and constipation (6) To report SUSPECTED ADVERSE REACTIONS, contact Eisai Inc. at (1-877-873-4724) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 9/2013 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
HALAVEN is indicated for the treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose of HALAVEN is 1.4 mg/m2 administered intravenously over 2 to 5 minutes on Days 1 and 8 of a 21-day cycle.
The recommended dose of HALAVEN in patients with mild hepatic impairment (Child-Pugh A) is 1.1 mg/m2 administered intravenously over 2 to 5 minutes on Days 1 and 8 of a 21-day cycle. [see Use in Specific Populations (8.6)].
The recommended dose of HALAVEN in patients with moderate hepatic impairment (Child-Pugh B) is 0.7 mg/m2 administered intravenously over 2 to 5 minutes on Days 1 and 8 of a 21-day cycle. [see Use in Specific Populations (8.6)].
The recommended dose of HALAVEN in patients with moderate renal impairment (creatinine clearance of 30-50 mL/min) is 1.1 mg/m2 administered intravenously over 2 to 5 minutes on Days 1 and 8 of a 21-day cycle. [see Use in Specific Populations (8.7)].
2.2 Dose Modification
Assess for peripheral neuropathy and obtain complete blood cell counts prior to each dose.
Recommended dose delays
- Do not administer HALAVEN on Day 1 or Day 8 for any of the following:
- ANC < 1,000/mm3
- Platelets < 75,000/mm3
- Grade 3 or 4 non-hematological toxicities. - The Day 8 dose may be delayed for a maximum of 1 week.
- If toxicities do not resolve or improve to ≤ Grade 2 severity by Day 15, omit the dose.
- If toxicities resolve or improve to ≤ Grade 2 severity by Day 15, administer HALAVEN at a reduced dose and initiate the next cycle no sooner than 2 weeks later.
Recommended dose reductions
- If a dose has been delayed for toxicity and toxicities have recovered to Grade 2 severity or less, resume HALAVEN at a reduced dose as set out in Table 1.
- Do not re-escalate HALAVEN dose after it has been reduced.
| Event Description |
Recommended HALAVEN Dose |
|---|---|
|
ANC = absolute neutrophil count. Toxicities graded in accordance with National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. |
|
| Permanently reduce the 1.4 mg/m2 HALAVEN dose for any of the following: | 1.1 mg/m2 |
| ANC < 500/mm3 for >7 days | |
| ANC < 1,000/mm3 with fever or infection | |
| Platelets < 25,000/mm3 | |
| Platelets < 50,000/mm3 requiring transfusion | |
| Non-hematologic Grade 3 or 4 toxicities | |
| Omission or delay of Day 8 HALAVEN dose in previous cycle for toxicity | |
| Occurrence of any event requiring permanent dose reduction while receiving 1.1 mg/m2 | 0.7 mg/m2 |
| Occurrence of any event requiring permanent dose reduction while receiving 0.7 mg/m2 | Discontinue HALAVEN |
2.3 Instructions for Preparation and Administration
Aseptically withdraw the required amount of HALAVEN from the single-use vial and administer undiluted or diluted in 100 mL of 0.9% Sodium Chloride Injection, USP.
Do not dilute in or administer through an intravenous line containing solutions with dextrose. Do not administer in the same intravenous line concurrent with the other medicinal products.
Store undiluted HALAVEN in the syringe for up to 4 hours at room temperature or for up to 24 hours under refrigeration (40°F or/ 4°C). Store diluted solutions of HALAVEN for up to 4 hours at room temperature or up to 24 hours under refrigeration.
Discard unused portions of the vial.
5 WARNINGS AND PRECAUTIONS
5.1 Neutropenia
Severe neutropenia (ANC < 500/mm3) lasting more than one week occurred in 12% (62/503) of patients in Study 1, leading to discontinuation in < 1% of patients [see Adverse Reactions (6)]. Patients with alanine aminotransferase or aspartate aminotransferase > 3 × ULN (upper limit of normal) experienced a higher incidence of Grade 4 neutropenia and febrile neutropenia than patients with normal aminotransferase levels. Patients with bilirubin > 1.5 × ULN also had a higher incidence of Grade 4 neutropenia and febrile neutropenia.
Monitor complete blood counts prior to each dose; increase the frequency of monitoring in patients who develop Grade 3 or 4 cytopenias. Delay administration of HALAVEN and reduce subsequent doses in patients who experience febrile neutropenia or Grade 4 neutropenia lasting longer than 7 days [see Dosage and Administration (2.2)]. Clinical studies of HALAVEN did not include patients with baseline neutrophil counts below 1,500/mm3.
5.2 Peripheral Neuropathy
Grade 3 peripheral neuropathy occurred in 8% (40/503) of patients, and Grade 4 in 0.4% (2/503) of patients in Study 1. Peripheral neuropathy was the most common toxicity leading to discontinuation of HALAVEN (5% of patients; 24/503). Neuropathy lasting more than one year occurred in 5% (26/503) of patients. Twenty-two percent (109/503) of patients developed a new or worsening neuropathy that had not recovered within a median follow-up duration of 269 days (range 25-662 days). Monitor patients closely for signs of peripheral motor and sensory neuropathy. Withhold HALAVEN in patients who experience Grade 3 or 4 peripheral neuropathy until resolution to Grade 2 or less [see Dosage and Administration (2.2)].
5.3 Embryo-Fetal Toxicity
There are no adequate and well-controlled studies of HALAVEN in pregnant women. HALAVEN is a microtubule inhibitor; therefore, it is expected to cause fetal harm when administered to a pregnant woman. Embryo-fetal toxicity and teratogenicity occurred in rats that received eribulin mesylate at approximately half of the recommended human dose based on body surface area. If this drug is used during pregnancy, or if a patient becomes pregnant while taking this drug, she should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
5.4 QT Prolongation
In an uncontrolled open-label ECG study in 26 patients, QT prolongation was observed on Day 8, independent of eribulin concentration, with no QT prolongation observed on Day 1. ECG monitoring is recommended if therapy is initiated in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, including Class Ia and III antiarrhythmics, and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating HALAVEN and monitor these electrolytes periodically during therapy. Avoid HALAVEN in patients with congenital long QT syndrome.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
The following adverse reactions are discussed in detail in other sections of the labeling:
- Neutropenia [see Warnings and Precautions (5.1)].
- Peripheral neuropathy [see Warnings and Precautions (5.2)].
- QT interval prolongation [see Warnings and Precautions (5.4)].
The most common adverse reactions (≥25%) reported in patients receiving HALAVEN were neutropenia, anemia, asthenia/fatigue, alopecia, peripheral neuropathy, nausea, and constipation. The most common serious adverse reactions reported in patients receiving HALAVEN were febrile neutropenia (4%) and neutropenia (2%). The most common adverse reaction resulting in discontinuation of HALAVEN was peripheral neuropathy (5%).
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
In clinical trials, HALAVEN has been administered to 1,222 patients with multiple tumor types, including 240 patients exposed to HALAVEN for 6 months or longer. The majority of the 1,222 patients were women (82%) with a median age of 58 years (range: 26 to 91 years). The racial and ethnic distribution was Caucasian (83%), Black (5%), Asian (2%), and other (5%).
The adverse reactions described in Table 2 were identified in 750 patients treated in Study 1 [see Clinical Studies (14)]. In Study 1, patients were randomized (2:1) to receive either HALAVEN (1.4 mg/m2 on Days 1 and 8 of a 21-day cycle) or single agent treatment chosen by their physician (control group). A total of 503 patients received HALAVEN, and 247 patients in the control group received therapy consisting of chemotherapy [total 97% (anthracyclines 10%, capecitabine 18%, gemcitabine 19%, taxanes 15%, vinorelbine 25%, other chemotherapies 10%)] or hormonal therapy (3%). The median duration of exposure was 118 days for patients receiving HALAVEN and 63 days for patients receiving control therapy. Table 2 reports the most common adverse reactions occurring in at least 10% of patients in either group.
|
MedDRA ver 10.0 |
HALAVEN n=503 |
Control Group n=247 |
||
| All Grades | ≥ Grade 3 | All Grades | ≥ Grade 3 | |
| Blood and Lymphatic System Disorders a | ||||
|
Neutropenia |
82% |
57% |
53% |
23% |
|
Anemia |
58% |
2% |
55% |
4% |
| Nervous system disorders | ||||
|
Peripheral neuropathyb |
35% |
8% |
16% |
2% |
|
Headache |
19% |
<1% |
12% |
<1% |
| General disorders and administrative site conditions | ||||
|
Asthenia/Fatigue |
54% |
10% |
40% |
11% |
|
Mucosal inflammation |
9% |
1% |
10% |
2% |
|
Pyrexia |
21% |
<1% |
13% |
<1% |
| Gastrointestinal disorders | ||||
|
Constipation |
25% |
1% |
21% |
1% |
|
Diarrhea |
18% |
0 |
18% |
0 |
|
Nausea |
35% |
1% |
28% |
3% |
|
Vomiting |
18% |
1% |
18% |
1% |
| Musculoskeletal and connective tissue disorders | ||||
|
Arthralgia/Myalgia |
22% |
<1% |
12% |
1% |
|
Back pain |
16% |
1% |
7% |
2% |
|
Bone pain |
12% |
2% |
9% |
2% |
|
Pain in extremity |
11% |
1% |
10% |
1% |
| Investigations | ||||
|
Weight decreased |
21% |
1% |
14% |
<1% |
| Metabolism and nutrition disorders | ||||
|
Anorexia |
20% |
1% |
13% |
1% |
| Respiratory, thoracic, and mediastinal disorders | ||||
|
Cough |
14% |
0 |
9% |
0 |
|
Dyspnea |
16% |
4% |
13% |
4% |
| Skin and subcutaneous tissue disorders | ||||
|
Alopecia |
45% |
NAc |
10% |
NAc |
| Infections and Infestations | ||||
|
Urinary Tract Infection |
10% |
1% |
5% |
0 |
|
a. based upon laboratory data. |
||||
Cytopenias: Grade 3 neutropenia occurred in 28% (143/503) of patients who received HALAVEN in Study 1, and 29% (144/503) of patients experienced Grade 4 neutropenia. Febrile neutropenia occurred in 5% (23/503) of patients; two patients (0.4%) died from complications of febrile neutropenia. Dose reduction due to neutropenia was required in 12% (62/503) of patients and discontinuation was required in <1% of patients. The mean time to nadir was 13 days and the mean time to recovery from severe neutropenia (<500/mm3) was 8 days. Grade 3 or greater thrombocytopenia occurred in 1% (7/503) of patients. G-CSF (granulocyte colony-stimulating factor) or GM-CSF (granulocyte–macrophage colony-stimulating factor) was used in 19% of patients who received HALAVEN.
Peripheral Neuropathy: In Study 1, 17 % of enrolled patients had Grade 1 peripheral neuropathy and 3% of patients had Grade 2 peripheral neuropathy at baseline. Dose reduction due to peripheral neuropathy was required by 3% (14/503) of patients who received HALAVEN. Four percent (20/503) of patients experienced peripheral motor neuropathy of any grade and 2% (8/503) of patients developed Grade 3 peripheral motor neuropathy.
Liver Function Test Abnormalities: Among patients with Grade 0 or 1 ALT levels at baseline, 18% of HALAVEN-treated patients experienced Grade 2 or greater ALT elevation. One HALAVEN-treated patient without documented liver metastases had concomitant Grade 2 elevations in bilirubin and ALT; these abnormalities resolved and did not recur with re-exposure to HALAVEN.
Less Common Adverse Reactions:The following additional adverse reactions were reported in ≥5% to <10% of the HALAVEN-treated group:
- Eye Disorders: increased lacrimation
- Gastrointestinal Disorders: dyspepsia, abdominal pain, stomatitis, dry mouth
- General Disorders and Administration Site Conditions: peripheral edema
- Infections and Infestations: upper respiratory tract infection
- Metabolism and Nutrition Disorders: hypokalemia
- Musculoskeletal and Connective Tissue Disorders: muscle spasms, muscular weakness
- Nervous System Disorders: dysgeusia, dizziness
- Psychiatric Disorders: insomnia, depression
- Skin and Subcutaneous Tissue Disorders: rash
6.2 Postmarketing Experience
The following adverse drug reactions have been identified during post-approval of HALAVEN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: lymphopenia
- Gastrointestinal Disorders: pancreatitis
- Hepatobiliary Disorders: hepatitis
- Immune System Disorders: drug hypersensitivity
- Infections and Infestations: pneumonia, sepsis/neutropenic sepsis
- Metabolism and Nutrition Disorders: hypomagnesemia, dehydration
- Respiratory, thoracic and mediastinal disorders: interstitial lung disease
- Psychiatric Disorders: anxiety
- Skin and Subcutaneous Tissue Disorders: pruritus
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on HALAVEN
No drug-drug interactions are expected with CYP3A4 inhibitors, CYP3A4 inducers or P-glycoprotein (P-gp) inhibitors. Clinically meaningful differences in exposure (AUC) were not observed in patients with advanced solid tumors when HALAVEN was administered with or without ketoconazole (a strong inhibitor of CYP3A4 and a P-gp inhibitor) and when HALAVEN was administered with or without rifampin (a CYP3A4 inducer) [see Clinical Pharmacology (12.3)].
7.2 Effects of HALAVEN on Other Drugs
Eribulin does not inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 or CYP3A4 enzymes or induce CYP1A2, CYP2C9, CYP2C19 or CYP3A4 enzymes at relevant clinical concentrations. Eribulin is not expected to alter the plasma concentrations of drugs that are substrates of these enzymes [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Category D [see Warnings and Precautions (5.3)]
There are no adequate and well-controlled studies with HALAVEN in pregnant women. HALAVEN is a microtubule inhibitor, therefore, it is expected to cause fetal harm when administered to a pregnant woman. Embryo-fetal toxicity and teratogenicity occurred in rats that received eribulin mesylate at approximately half of the recommended human dose based on body surface area. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
In a developmental toxicity study, pregnant rats received intravenous infusion of eribulin mesylate during organogenesis (Gestation Days 8, 10, and 12) at doses approximately 0.04, 0.13, 0.43 and 0.64 times the recommended human dose, based on body surface area (mg/m2). Increased abortion and severe external or soft tissue malformations were observed in offspring at doses 0.64 times the recommended human dose based on body surface area (mg/m2), including the absence of a lower jaw, tongue, stomach and spleen. Increased embryo-fetal death/resorption, reduced fetal weights, and minor skeletal anomalies consistent with developmental delay were also reported at or above doses of 0.43 times the recommended human dose.
Maternal toxicity of eribulin mesylate was reported in rats at or above doses of 0.43 times the recommended human dose (mg/m2), and included enlarged spleen, reduced maternal weight gain and decreased food consumption.
8.3 Nursing Mothers
It is not known whether HALAVEN is excreted into human milk. No studies in humans or animals were conducted to determine if HALAVEN is excreted into milk. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in human milk fed infants from HALAVEN, a decision should be made whether to discontinue nursing or to discontinue HALAVEN taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of HALAVEN in pediatric patients below the age of 18 years have not been established.
8.5 Geriatric Use
Study 1 did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. Of the 827 subjects who received the recommended dose and schedule of HALAVEN in clinical studies, 15% (121/827) were 65 and older, and 2% (17/827) patients were 75 and older. No overall differences in safety were observed between these subjects and younger subjects.
8.6 Hepatic Impairment
Administration of HALAVEN at a dose of 1.1 mg/m2 to patients with mild hepatic impairment and 0.7 mg/m2 to patients with moderate hepatic impairment resulted in similar exposure to eribulin as a dose of 1.4 mg/m2 to patients with normal hepatic function. Therefore, a lower starting dose of 1.1 mg/m2 is recommended for patients with mild hepatic impairment (Child-Pugh A) and of 0.7 mg/m2 is recommended for patients with moderate hepatic impairment (Child-Pugh B). HALAVEN was not studied in patients with severe hepatic impairment (Child-Pugh C). [see Dosage and Administration (2.1), Clinical Pharmacology (12.3)]
8.7 Renal Impairment
For patients with moderate renal impairment (CrCl 30-50 mL/min), the geometric mean dose-normalized systemic exposure increased 2-fold compared to patients with normal renal function. A lower starting dose of 1.1 mg/m2 is recommended for patients with moderate renal impairment. The safety of HALAVEN was not studied in patients with severe renal impairment (CrCl < 30 mL/min). [see Dosage and Administration (2.1), Clinical Pharmacology (12.3)]
10 OVERDOSAGE
Overdosage of HALAVEN has been reported at approximately 4 times the recommended dose, which resulted in Grade 3 neutropenia lasting seven days and a Grade 3 hypersensitivity reaction lasting one day.
There is no known antidote for HALAVEN overdose.
11 DESCRIPTION
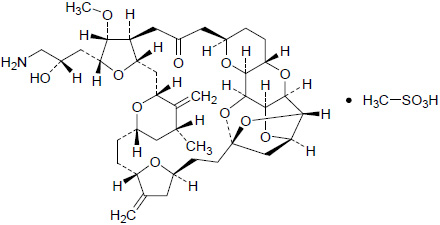
HALAVEN (eribulin mesylate) Injection is a non-taxane microtubule dynamics inhibitor. Eribulin mesylate is a synthetic analogue of halichondrin B, a product isolated from the marine sponge Halichondria okadai. The chemical name for eribulin mesylate is 11,15:18,21:24,28-Triepoxy-7,9-ethano-12,15-methano-9H,15H-furo[3,2-i]furo[2',3':5,6]pyrano[4,3-b][1,4]dioxacyclopentacosin-5(4H)-one, 2-[(2S)-3-amino-2-hydroxypropyl]hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)-, (2R,3R,3aS,7R,8aS,9S,10aR,11S,12R,13aR,13bS,15S,18S,21S,24S,26R,28R,29aS)-, methanesulfonate (salt). It has a molecular weight of 826.0 (729.9 for free base). The empirical formula is C40H59NO11•CH4O3S. Eribulin mesylate has the following structural formula:
HALAVEN is a clear, colorless, sterile solution for intravenous administration. Each vial contains 1 mg of eribulin mesylate as a 0.5 mg/mL solution in ethanol: water (5:95).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Eribulin inhibits the growth phase of microtubules without affecting the shortening phase and sequesters tubulin into nonproductive aggregates. Eribulin exerts its effects via a tubulin-based antimitotic mechanism leading to G2/M cell-cycle block, disruption of mitotic spindles, and, ultimately, apoptotic cell death after prolonged mitotic blockage.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of eribulin is linear with a mean elimination half-life of approximately 40 hours, a mean volume of distribution of 43 L/m2 to 114 L/m2 and mean clearance of 1.16 L/hr/m2 to 2.42 L/hr/m2 over the dose range of 0.25 mg/m2 to 4.0 mg/m2. The human plasma protein binding of eribulin at concentrations of 100 ng/mL to 1,000 ng/mL ranges from 49% to 65%. Eribulin exposure after multiple dosing is comparable to that following a single dose. No accumulation of eribulin is observed with weekly administration.
Metabolism
Unchanged eribulin was the major circulating species in plasma following administration of 14C-eribulin to patients. Metabolite concentrations represented < 0.6% of parent compound, confirming that there are no major human metabolites of eribulin. Cytochrome P450 3A4 (CYP3A4) negligibly metabolizes eribulin in vitro.
Elimination
Eribulin is eliminated primarily in feces unchanged. After administration of 14C-eribulin to patients, approximately 82% of the dose was eliminated in feces and 9% in urine. Unchanged eribulin accounted for approximately 88% and 91% of the dose in feces and urine, respectively.
Effects of Age, Gender, and Race
Based on a population pharmacokinetic analysis with data collected from 340 patients, gender, race, and age do not have a clinically meaningful effect on the PK of eribulin.
Drug Interactions
Effect of Other Drugs on HALAVEN
The effect of a strong CYP3A4 inhibitor and a P-gp inhibitor, ketoconazole, on the PK of eribulin was studied in a crossover trial of 12 patients with advanced solid tumors. No clinically relevant PK interaction was observed when HALAVEN was administered with or without ketoconazole (the geometric mean ratio of the AUC: 0.97; 90%CI: 0.83, 1.12).
The effect of a CYP3A4 inducer, rifampin, on the PK of eribulin was studied in a crossover trial of 14 patients with advanced solid tumors. No clinically relevant PK interaction was observed when HALAVEN was administered with or without rifampin (the geometric mean ratio of the AUC: 1.10; 90CI%: 0.91, 1.34).
Effect of HALAVEN on Other Drugs
Eribulin shows no induction potential for CYP1A, CYP2C9, CYP2C19, and CYP3A in primary human hepatocytes. Eribulin inhibits CYP3A4 activity in human liver microsomes, but it is unlikely that eribulin will substantially increase the plasma levels of CYP3A4 substrates. No significant inhibition of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP2E1 was detected with eribulin concentrations up to 5 μM in pooled human liver microsomes. In vitro drug interaction studies indicate that eribulin does not inhibit drugs that are substrates of these enzymes and it is unlikely that eribulin will affect plasma levels of drugs that are substrates of CYP enzymes. Eribulin is a substrate and a weak inhibitor of the drug efflux transporter P-gp in vitro.
Specific Populations
Hepatic Impairment
A study evaluated the PK of eribulin in patients with mild (Child-Pugh A; n=7) and moderate (Child-Pugh B; n=5) hepatic impairment. Compared to patients with normal hepatic function (n=6), eribulin exposure increased 1.8-fold and 2.5-fold in patients with mild and moderate hepatic impairment, respectively. Administration of HALAVEN at a dose of 1.1 mg/m2 to patients with mild hepatic impairment and 0.7 mg/m2 to patients with moderate hepatic impairment resulted in similar exposure to eribulin as a dose of 1.4 mg/m2 to patients with normal hepatic function. [see Dosage and Administration (2.1), Use in Specific Populations (8.6)]
Renal Impairment
No formal PK trials were conducted with HALAVEN in patients with renal impairment. Available data suggests that geometric mean dose-normalized systemic exposure is similar for patients with mild renal impairment (CrCl 50-80 mL/min) relative to patients with normal renal function. However, for patients with moderate renal impairment (CrCl 30-50 mL/min), the geometric mean dose-normalized systemic exposure increased 2-fold compared to patients with normal renal function. [see Dosage and Administration (2.1), Use in Specific Populations (8.7)]
12.6 Cardiac Electrophysiology
The effect of HALAVEN on the QTc interval was assessed in an open-label, uncontrolled, multicenter, single-arm dedicated QT trial. A total of 26 patients with solid tumors received 1.4 mg/m2 of HALAVEN on Days 1 and 8 of a 21-day cycle. A delayed QTc prolongation was observed on Day 8, with no prolongation observed on Day 1. The maximum mean QTcF change from baseline (95% upper confidence interval) was 11.4 (19.5) ms.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, mutagenesis, impairment of fertility
Carcinogenicity studies have not been conducted with eribulin mesylate.
Eribulin mesylate was not mutagenic in in vitro bacterial reverse mutation assays (Ames test). Eribulin mesylate was positive in mouse lymphoma mutagenesis assays, and was clastogenic in an in vivo rat bone marrow micronucleus assay.
The effects of HALAVEN on human fertility are unknown. Fertility studies have not been conducted with eribulin mesylate in humans or animals. However, nonclinical findings in repeated-dose dog and rat toxicology studies suggest that male fertility may be compromised by treatment with eribulin mesylate. Rats exhibited testicular toxicity (hypocellularity of seminiferous epithelium with hypospermia/aspermia) following dosing with eribulin mesylate at or above 0.43 times the recommended human dose (mg/m2) given once weekly for 3 weeks, or at or above 0.21 times the recommended human dose (mg/m2) given once weekly for 3 out of 5 weeks, repeated for 6 cycles. Testicular toxicity was also observed in dogs given 0.64 times the recommended human dose (mg/m2) weekly for 3 out of 5 weeks, repeated for 6 cycles.
14 CLINICAL STUDIES
Study 1 was an open-label, randomized, multicenter trial of 762 patients with metastatic breast cancer who received at least two chemotherapeutic regimens for the treatment of metastatic disease and experienced disease progression within 6 months of their last chemotherapeutic regimen. Patients were required to receive prior anthracycline- and taxane-based chemotherapy for adjuvant or metastatic disease. Patients were randomized (2:1) to receive HALAVEN (n=508) or a single agent therapy selected prior to randomization (control arm, n=254). Randomization was stratified by geographic region, HER2/neu status, and prior capecitabine exposure. HALAVEN was administered at a dose of 1.4 mg/m2 on Days 1 and 8 of a 21-day cycle. HALAVEN-treated patients received a median of 5 cycles (range: 1 to 23 cycles) of therapy. Control arm therapy consisted of 97% chemotherapy (26% vinorelbine, 18% gemcitabine, 18% capecitabine, 16% taxane, 9% anthracycline, 10% other chemotherapy), and 3% hormonal therapy. The main efficacy outcome was overall survival.
Patient demographic and baseline characteristics were comparable between the treatment arms. The median age was 55 (range: 27 to 85 years) and 92% were White. Sixty-four percent of patients were enrolled in North America/Western Europe/Australia, 25% in Eastern Europe/Russia, and 11% in Latin America/South Africa. Ninety-one percent of patients had a baseline ECOG performance status of 0 or 1. Tumor prognostic characteristics, including estrogen receptor status (positive: 67%, negative: 28%), progesterone receptor status (positive: 49%, negative: 39%), HER2/neu receptor status (positive: 16%, negative: 74%), triple negative status (ER-, PR-, HER2/neu-: 19%), presence of visceral disease (82%, including 60% liver and 38% lung) and bone disease (61%), and number of sites of metastases (greater than two: 50%), were also similar in the HALAVEN and control arms. Patients received a median of four prior chemotherapy regimens in both arms.
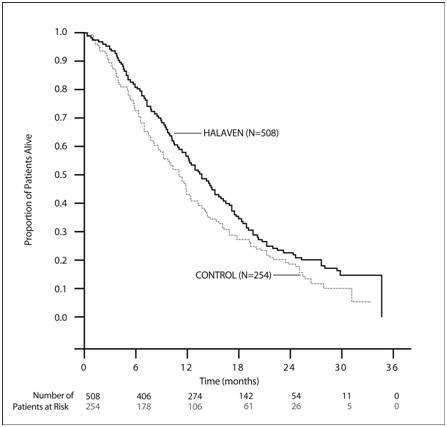
In Study 1, a statistically significant improvement in overall survival was observed in patients randomized to the HALAVEN arm compared to the control arm (see Table 3). An updated, unplanned survival analysis, conducted when 77% of events had been observed (see Figure 1), was consistent with the primary analysis. In patients randomized to HALAVEN, the objective response rate by the RECIST criteria was 11% (95% CI: 8.6%, 14.3%) and the median response duration was 4.2 months (95% CI: 3.8, 5.0 months).
| Overall Survival | HALAVEN (n=508) | Control Arm (n=254) |
|---|---|---|
| CI = confidence interval a Based on Cox proportional hazards model stratified by geographic region, HER2 status, and prior capecitabine therapy. b Based on a log-rank test stratified by geographic region, HER2 status, and prior capecitabine therapy. |
||
| Primary survival analysis | ||
| Number of deaths | 274 | 148 |
| Median, months (95% CI) | 13.1 (11.8, 14.3) | 10.6 (9.3, 12.5) |
| Hazard Ratio (95% CI)a | 0.81 (0.66, 0.99) | |
| P valueb | 0.041 | |
| Updated survival analysis | ||
| Number of deaths | 386 | 203 |
| Median, months (95% CI) | 13.2 (12.1, 14.4) | 10.6 (9.2, 12.0) |
Figure 1 Updated Overall Survival Analysis for Study 1
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC 62856-389-01 Eribulin mesylate injection, 1 mg/2 mL, in a single-use vial. One vial per carton.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). Do not freeze. Store the vials in their original cartons.
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling
- Advise patients to contact their health care provider for a fever of 100.5°F or greater or other signs or symptoms of infection such as chills, cough, or burning or pain on urination. [see Warnings and Precautions (5.1)]
- Advise women of childbearing potential to avoid pregnancy and to use effective contraception during treatment with HALAVEN. [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1)]
Distributed by:
Eisai Inc.
Woodcliff Lake, NJ 07677
© 2013 Eisai Inc.
Revised September 2013
PATIENT INFORMATION
HALAVEN® (HAL-ih-ven)
(eribulin mesylate) Injection
Read this leaflet before you start receiving HALAVEN and before each injection. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about HALAVEN?
Your healthcare provider should do blood tests regularly to check your blood cell counts before you receive each dose of HALAVEN.
- HALAVEN can cause a decrease in white blood cell count (neutropenia). This can make you more likely to get serious infections that could lead to death. You may need treatment in the hospital with antibiotic medicines.
- Call your healthcare provider right away if you develop any of these symptoms of infection while you are receiving HALAVEN:
- fever (temperature above 100.5°F)
- chills
- cough
- burning or pain when you urinate.
- HALAVEN can cause numbness, tingling, or burning in your hands and feet (neuropathy). Tell your healthcare provider if you have any of these symptoms.
See "What are possible side effects of HALAVEN?" for more information about side effects.
What is HALAVEN?
HALAVEN is a prescription medicine used to treat people with breast cancer:
- that has spread to other parts of their body, and
- who have already received certain types of anticancer medicines after their breast cancer has spread.
What should I tell my healthcare provider before receiving HALAVEN?
Before you receive HALAVEN, tell your healthcare provider if you:
- have liver or kidney problems.
- have heart problems, including a problem called "congenital long QT syndrome."
- are pregnant or plan to become pregnant. HALAVEN may harm your unborn baby. Talk with your healthcare provider about birth control methods to prevent pregnancy while you receive HALAVEN. Tell your healthcare provider right away if you become pregnant or think you are pregnant while you are receiving HALAVEN
- are breastfeeding or planning to breastfeed. It is not known if HALAVEN passes into your breast milk. You and your healthcare provider should decide if you will take HALAVEN or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
Know the medicines you take. Keep a list of your medicines to show to your healthcare provider and pharmacist when you get a new medicine.
How will I receive HALAVEN?
- HALAVEN is injected directly into your vein.
- HALAVEN is given in "cycles" of treatment, with each cycle lasting 21 days.
- You will receive an injection 1 time each week for two weeks in a row (day 1 and day 8 of a treatment cycle).
- Your healthcare provider may need to decrease your dose of HALAVEN or change how often you receive it, depending on your blood test results.
What are the possible side effects of HALAVEN?
HALAVEN may cause serious side effects, including:
- See "What is the most important information I should know about HALAVEN?"
- HALAVEN can cause changes in your heartbeat (called QTc prolongation). This can cause irregular heartbeats that may lead to death. Your healthcare provider will decide if you need heart monitoring (electrocardiogram or ECG), or blood tests during your treatment with HALAVEN to watch for this problem.
The most common side effects of HALAVEN include:
- weakness or tiredness
- hair loss
- nausea
- constipation
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of HALAVEN. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about HALAVEN
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. This leaflet summarizes the most important information about HALAVEN. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about HALAVEN that is written for health professionals.
For more information, go to www.HALAVEN.com or call Eisai Inc. at 1-877-873-4724.
What are the ingredients in HALAVEN?
Active Ingredients: eribulin mesylate
Inactive Ingredients: ethanol, water
HALAVEN® is a registered trademark used by Eisai Inc. under license from Eisai R&D Management Co., Ltd.
Distributed by:
Eisai Inc.
Woodcliff Lake, NJ 07677
If you would like a leaflet with larger printing, please contact Eisai Inc. at 1-877-873-4724.
Revised: September 2013
| HALAVEN
eribulin mesylate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Eisai Inc. (831600833) |