IOBENGUANE SULFATE I 131 INJECTION
-
iobenguane i-131 injection
Pharmalucence, Inc.
----------
Iobenguane Sulfate I 131 InjectionDiagnostic - For Intravenous Use
DESCRIPTION
Iobenguane Sulfate I 131 Injection is a sterile, pyrogen free radiopharmaceutical for intravenous injection. Each milliliter contains 0.69 mg of iobenguane sulfate, 85.1 MBq (2.30 mCi) of I 131 (as iobenguane sulfate I 131 at calibration), 0.36 mg of sodium acetate, 0.27 mg of acetic acid, 4.2 mg of sodium chloride, 0.56 mg of methyl paraben, 0.056 mg of propylparaben and 0.01 mL of benzyl alcohol. Iobenguane Sulfate I 131 is also known as I 131-meta-iodobenzylguanidine sulfate (I 131 MIBG) and has the following structural formula:
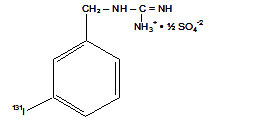
Iobenguane Sulfate I 131
(I 131-meta-iodobenzylguanidine sulfate)
Physical Characteristics
Iobenguane Sulfate I 131 is a radioiodinated arylalkylguanidine. It is similar in structure to the anti-hypertensive drug guanethidine and to the neurotransmitter norepinephrine.
Iodine 131 decays by beta and gamma emission and has a physical half-life of 8.04 days. The principal beta particles and those photons that are useful for detection and imaging are listed in Table 1.
| Radiation | Mean Percentage Disintegration | Energy (kev) |
| Beta-1 | 2.1 | 69.4 avg |
| Beta-3 | 7.4 | 96.6 avg |
| Beta-4 | 89.3 | 191.6 avg |
| Gamma-7 | 6.14 | 284.3 |
| Gamma-14 | 81.7 | 364.5 |
| Gamma-17 | 7.17 | 637.0 |
External Radiation
The specific gamma ray constant for I 131 is 2.2 R/mCi/hr at 1cm. The first half-value thickness of lead (Pb) for I 131 is 0.24 cm. The relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2; i.e. the use of 2.55 cm of Pb will decrease the external radiation exposure by a factor of about 1,000.
|
|
| Shield Thickness (Pb) cm | Coefficient of Attenuation |
| 0.24 | 0.5 |
| 0.89 | 10-1 |
| 1.60 | 10-2 |
| 2.55 | 10-3 |
| 3.73 | 10-4 |
| Days | Activity x Calibration | Days | Activity x Calibration |
| -14 | 3.343 | 1 | 0.917 |
| -13 | 3.067 | 2 | 0.842 |
| -12 | 2.814 | 3 | 0.772 |
| -11 | 2.581 | 4 | 0.708 |
| -10 | 2.368 | 5 | 0.650 |
| -9 | 2.173 | 6 | 0.596 |
| -8 | 1.993 | 7 | 0.547 |
| -7 | 1.828 | 8 | 0.502 |
| -6 | 1.677 | 9 | 0.460 |
| -5 | 1.539 | 10 | 0.422 |
| -4 | 1.412 | 11 | 0.387 |
| -3 | 1.295 | 12 | 0.355 |
| -2 | 1.188 | 13 | 0.326 |
| -1 | 1.090 | 14 | 0.299 |
| Calibration Date | 1.000 |
CLINICAL PHARMACOLOGY
General
Iobenguane Sulfate I 131 enters adrenergic neurons and chromaffin cells primarily by the type I (active transport) mechanism of catecholamine uptake into adrenergic storage granules. Its uptake is blocked by drugs which interfere with catecholamine uptake (see drug interaction section). Within about 2 hours, 80% of Iobenguane Sulfate I 131 distributes from plasma to erythrocytes and body tissues. After background clearance, visualization of abnormal adrenal medullary tissue peaks at about 48 hours post-injection. Normal adrenal glands are seen faintly in 2% of patients. Normal salivary glands, liver, spleen, and urinary bladder may also be seen to a lesser extent. Excretion is primarily by the kidneys.
Pharmacokinetics
The pharmacokinetics profile of Iobenguane Sulfate I 131 fits a 3 compartment model. The physical half-life of I 131 is 8.04 days. The maximum biologic half-life of Iobenguane Sulfate I 131 (including metabolites), computed by the Sigma minus method from urinary excretion data from patients with normal renal function, is about 5 days.
Metabolism
In patients with normal renal function, the major metabolites that account for less than 10% of the administered dose are m-iodohippuric action (MIHA), m-iodobenzoic acid (MIBA) and 4-hydroxy-3-iodobenzylguanidine (HIBG) and radioiodide. The enzymatic process responsible for metabolism has not been well characterized and the pharmacologic activity of these metabolites has not been studied.
In patients with normal renal function, about 50% of the injected radioactivity was recovered in urine during the first 24 hours after the infusion. About 90% was recovered in the urine by 4 days, primarily as unchanged iobenguane. Elimination is relatively independent of dose from 0.5 mCi (0.15 mg) to approximately 213 mCi (5mg).
Pharmacodynamics
Iobenguane Sulfate I 131 localizes within intracellular adrenergic storage granules. Glomerular filtration is primarily responsible for extracellular clearance of Iobenguane Sulfate I 131 from the body. In a 192 hour study of an anephric patient, elimination was not noted. The formation of metabolites increases in patients with renal impairment and may increase in patients with substantial tumor burdens (e.g.; an extensively metabolizing pheochromocytoma). Elimination by other routes is not well characterized. Iobenguane Sulfate I 131 is not cleared by dialysis. Dosage adjustments in renally impaired patients have not been studied.
CLINICAL TRIALS
Three clinical trials were performed in a total of 397 evaluable patients with suspected pheochromocytoma. Of these subjects, 212 were males and 185 were females. The mean age was 46.3 years (range of 1-85 years). About two-thirds of the patients were between 31 and 60 years of age; 25 subjects were less than 20 years; 5 were less than 10 years of age. The mean weight of all subjects was 78.6 kg (range of 7.6-189 kgs).A racial distribution is not available.
Patients were entered into the study who, after consideration of their clinical history, physical examination and laboratory findings, were considered to have reasonable suspicion of having pheochromocytoma. Pregnant women were excluded. The diagnosis of pheochromocytoma was confirmed by other diagnostic procedures (plasma and urinary catecholamine, clonidine suppression tests and abdominal CT scans). Adult patients up to 65 kg received 0.5 mCi Iobenguane Sulfate I 131, those >65 kg received 0.3 mCi/m2. Children received 0.3 mCi/m2.
Based upon dosimetry calculation from biodistribution studies, the mean dose was 0.519 mCi (range 0.10 to 1.10 mCi). All subjects had their thyroid gland iodine uptake blocked with Potassium Iodide Oral Solution (120 mg KI/day = 0.12 mL/day) or Lugol’s Solution (up to 40 mg I/day = 0.3 mL/day). In diagnosing pheochromocytoma, Iobenguane Sulfate I 131 had an overall sensitivity of 71% and overall specificity rate of 94%. Within a subgroup of 293 patients who had the presence or absence of disease confirmed, there were 25 false negative and 7 false positive scans.
In further analysis of patients with confirmed pheochromocytoma, in patients who were not on medications that could potentially interfere with Iobenguane Sulfate I 131 uptake, the sensitivity was 83%; in patients on potentially interfering medications, the sensitivity was reduced to 52%. The specificity was not altered. This suggests that in previously undiagnosed pheochromocytoma patients, the concomitant use of potentially blocking medications may confound the scan results.
In another trial, 72 patients were studied for suspected neuroblastoma. Of these subjects, there was an equal gender distribution. The mean age was 4.3 years (range 0.3-27 years); 46 of the 72 patients were between 1 and 5 years of age, 3 patients were over 15 years, 11 patients were under 1 year of age. The mean weight was 19.2 kg (range 3.1-115.1 kgs). A racial distribution is not available.
Patients suspected clinically of having neuroblastoma were included in the study. Most patients had other diagnostic procedures (bone scans, CT scans, MRI, etc.) before they were asked to participate in the study. Critically ill patients were generally excluded. Adults up to 65 kg received 0.5 mCi, those >65 kg received 0.3 mCi/m2 Iobenguane Sulfate I 131. Children received 0.3 mCi/m2. Based on dosimetry calculations from biodistribution studies, the mean dose was 0.231 mCi (range from 0.063 to 1.031 mCi). All subjects received Potassium Iodide Oral Solution (120 mg KI/day = 0.12 mL/day) or Lugol’s Solution (up to 40 mg I/day = 0.3 mL/day) to block thyroid uptake. In this trial, Iobenguane Sulfate I 131 localized neuro-blastomas with an 85.1% sensitivity and 92.0% specificity. There were 4 false negative and 2 false positive scans. None of the children on these trials were on medication that could potentially interfere with the uptake of Iobenguane Sulfate I 131.
INDICATIONS AND USAGE
Iobenguane Sulfate I 131 Injection is indicated as an adjunctive diagnostic agent in the localization of primary or metastatic pheochromocytomas and neuroblastomas.
GERIATRIC USE
Clinical studies of Iobenguane Sulfate I 131 did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
CONTRAINDICATIONS
Iobenguane Sulfate I 131 is contraindicated in patients with known hypersensitivity to iobenguane sulfate.
WARNINGS
As with other I 131 containing agents, in order to decrease thyroid accumulation of I 131, block the thyroid gland with iodine. (See DOSAGE AND ADMINISTRATION section)
During and following the injection, patients with known or suspected pheochromocytoma should be carefully monitored for hypertensive crises.
PRECAUTIONS
General
IOBENGUANE SULFATE I 131 IS CLEARED BY GLOMERULAR FILTRATION AND IS NOT DIALYZABLE. Caution should be exercised when administering the drug to renally impaired patients. Iobenguane Sulfate I 131 is not recommended in anephric patients. The radiation dose to the anephric patient would be substantially increased due to the delayed biological elimination of the drug. Also, because of the lack of clearance, the target-to-background ratios would severely compromise the outcome of the study. Iobenguane Sulfate I 131 use in patients with impaired renal function should be carefully considered. As with all radio-iodinated compounds, the patient should be well hydrated before and during examination.
Although iodinated contrast imaging agents have been confirmed to cause anaphylactic reactions in patients with hypersensitivity to iodine, the incidence of hypersensitivity reactions to Iobenguane Sulfate I 131 is rare. Since hypersensitivity or immune reactions are not concentration dependent, emergency treatment measures should be available.
Cardiac
Electrocardiographic (ECG) changes have been documented in dogs after the administration of 18 times the mg/m2 conversion of the maximum human dose of Iobenguane Sulfate I 131. The maximum no observable effect level (NOEL) is not known. It is unknown if Iobenguane Sulfate I 131 can produce changes in ECG recordings in man.
Drug Interactions
There are literature reports about patients and about in-vitro systems which suggest that the following drugs have the potential to decrease uptake of Iobenguane Sulfate I 131 in neuro-endocrine tumors and may lead to false negative results if administered concomitantly: anti-hypertensives (labetalol, reserpine, calcium channel blockers), amitriptyline and derivatives, imipramine and derivatives, doxepin, amoxapin, and loxapin, sympathetic-amines (phenyl-ephrine, phenylpropanolamine, pseudoephedrine, ephedrine) and cocaine. The clinical studies were not designed to show which drugs could cause false negative results. It is unknown if other drugs in the same classes have the same potential to inhibit the uptake of Iobenguane Sulfate I 131. Increasing the dose of Iobenguane Sulfate I 131 will not overcome any potential uptake-limiting effect of these drugs.
Normal biodistribution and excretion of Iobenguane Sulfate I 131 leads to localization in adrenergic storage granules of the adrenal gland. It is also localized in salivary glands, liver, spleen and urinary bladder. As in all nuclear imaging procedures, careful positioning may be useful in distinguishing normal biodistribution of the agent from localization in sites of pathology.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with Iobenguane Sulfate I 131 have not been conducted to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy Category C
Animal reproduction studies have not been conducted with Iobenguane Sulfate I 131. It is also not known whether Iobenguane Sulfate I 131 can cause fetal harm when administered to a pregnant woman or if it can affect reproductive capacity. Therefore, Iobenguane Sulfate I 131 should not be administered to a pregnant woman unless the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
I 131 is excreted in human milk; it is not known if Iobenguane Sulfate I 131 is excreted in human milk. Therefore, breast feeding should be substituted with formula feeding until the Iobenguane Sulfate I 131 has cleared from the body of the nursing woman.
PEDIATRIC USE
The safety and effectiveness of Iobenguane Sulfate I 131 have been reasonably established in pediatric patients with neuroblastoma and pheochromocytoma.
Safety, effectiveness, metabolism, urinary excretion and tumor specificity of Iobenguane Sulfate I 131 is unknown in neonates.
ADVERSE REACTIONS
Transient episodes of marked hypertension have been reported in patients after injection of Iobenguane Sulfate I 131. Some of these patients were on anti-hypertensives and others were not.
Nausea, vomiting and sleepiness have been reported after injection of higher than the recommended doses of Iobenguane Sulfate I 131. The no effect level for these reactions has not been identified. An episode of fever, chills and hypotension has been reported. In clinical trials, no deaths have been attributed to the drug.
DOSAGE AND ADMINISTRATION
Before administration of Iobenguane Sulfate I 131, the patient’s thyroid gland should be blocked with Potassium Iodide Oral Solution (120 mg KI/day = 0.12 mL/day) or Lugol’s Solution (up to 40 mg I/day = 0.3 mL/day). The blocking iodine should be administered one day before and daily for 5 to 7 days after the dose of Iobenguane Sulfate I 131
Adults
The recommended dose in adults is 0.5 mCi. In obese patients over 1.7 m2 (65 kg), the dose should be 0.3 mCi/m2 up to a maximum of 1.0 mCi.
Pediatric Patients
The recommended dose in pediatric patients is 0.3 mCi/m2 up to a maximum total dose of 0.5 mCi. The minimum recommended dose for adequate imaging is 0.135 mCi.
Iobenguane Sulfate I 131 should be injected by slow intravenous infusion over 15-30 seconds (longer if necessary). Since the possibility of rebound hypertension exists, the patient’s vital signs should be carefully monitored during and after injection.
In order to maintain sterility, it is essential that the user follow directions and adhere to strict aseptic procedure. As in the use of any radioactive material, care should be taken to insure minimum radiation exposure to the patient and clinical personnel.
Waterproof gloves should be worn by the user and a shielded syringe should be used during the preparation and administration of the dose. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Radiation Dosimetry
The estimated absorbed radiation doses to adults and children from an intravenous dose of Iobenguane Sulfate I 131 are shown in Table 4.
| Organ | Adult | 15 Years | 10 years | 5 years | 1 years | Newborn | ||||||
|
mGy/ 37 MBq |
rads/ 1mCi |
mGy/ 18.5 MBq |
rads/ 0.5 mCi |
mGy/ 18.5 MBq |
rads/ 0.5 mCi |
mGy/ 18.5 MBq |
rads/ 0.5 mCi |
mGy/ 18.5 MBq |
rads/ 0.5 mCi |
mGy/ 18.5 MBq |
rads/ 0.5 mCi |
|
| Urinary | ||||||||||||
| Bladder | ||||||||||||
| Wall | 28.0 | 2.8 | 18.5 | 1.9 | 28.0 | 2.8 | 43.5 | 4.4 | 85.0 | 8.5 | 215.0 | 21.5 |
| Liver | 29.0 | 2.9 | 19.0 | 1.9 | 29.5 | 3.0 | 43.5 | 4.4 | 85.0 | 8.5 | 190.0 | 19.0 |
| Spleen | 22.0 | 2.2 | 15.5 | 1.6 | 24.5 | 2.5 | 38.5 | 3.9 | 70.0 | 7.0 | 195.0 | 19.5 |
| Heart Wall | 2.9 | 0.3 | 1.9 | 0.2 | 2.9 | 0.3 | 4.5 | 0.5 | 8.5 | 0.9 | 19.5 | 2.0 |
| Adrenals | 7.5 | 0.8 | 5.5 | 0.6 | 8.0 | 0.8 | 10.5 | 1.1 | 16.5 | 1.7 | 16.5 | 1.7 |
| Gallbladder | ||||||||||||
| Wall | 5.1 | 0.5 | 3.0 | 0.3 | 4.4 | 0.4 | 7.0 | 0.7 | 12.5 | 1.3 | 28.0 | 2.8 |
| Pancreas | 3.8 | 0.4 | 2.4 | 0.2 | 3.8 | 0.4 | 6.0 | 0.6 | 10.5 | 1.1 | 23.5 | 2.4 |
| Thyroid | 3.3 | 0.3 | 2.6 | 0.3 | 4.0 | 0.40 | 8.5 | 0.9 | 16.5 | 1.7 | 24.0 | 2.4 |
| Kidneys | 3.2 | 0.3 | 2.0 | 0.2 | 3.1 | 0.3 | 4.9 | 0.5 | 8.5 | 0.9 | 20.0 | 2.0 |
| Uterus | 3.3 | 0.3 | 2.1 | 0.2 | 3.3 | 0.3 | 5.0 | 0.5 | 9.5 | 1.0 | 22.0 | 2.2 |
| Ovaries | 2.7 | 0.3 | 1.8 | 0.2 | 2.8 | 0.3 | 4.4 | 0.4 | 8.5 | 0.9 | 19.5 | 2.0 |
| Testes | 2.2 | 0.2 | 1.4 | 0.1 | 2.3 | 0.2 | 3.7 | 0.4 | 7.0 | .7 | 17.5 | 1.8 |
| Brain | 1.7 | 0.2 | 1.1 | 0.1 | 1.9 | 0.2 | 3.1 | 0.3 | 6.0 | 0.6 | 15.0 | 1.5 |
|
Effective Dose Equivalent (rem) | 0.7 | 0.5 | 0.8 | 1.2 | 2.2 | 5.0 | ||||||
* Based on data gathered in patients – Jacobsson et al, 4 International Radiopharmaceutical
Dosimetry Symposium, CONF – 851113, pp. 389-398.
Estimate calculated using phantoms of Christy & Eckerman (Report ORNL/TM-8381/V1 & V7).
The effective dose equivalent is a quantity which may be suitable for comparing risks of different procedures in nuclear medicine, radiology, and other applications involving ionizing radiation, but should not be construed to give information about risks to individual patients and should not be applied to situations involving radiation therapy.
The following organs each receive less than 1 rad per adult procedure: breasts, LLI wall, small intestine, stomach, ULI wall, lungs, muscle, red marrow, bone surfaces, skin and thymus.
If 0.5 mCi of Iobenguane Sulfate I 131 is used for an adult dose, the organ burden would be half of the doses listed above. The thyroid gland estimated burden is in the unblocked state. When the thyroid gland is blocked with Lugol’s solution, uptake in minimal.
Peak scans were generally noted at 48 hours post-injection. However, serial scans at 24, 48 and 72 hours post-injection may be needed to optimally define the tumor.
HOW SUPPLIED
Each 2 ml glass vial contains a total volume of 0.5 mL with a total activity of, at calibration time,
42.6 MBq (1.15 mCi) of Iobenguane Sulfate I 131 Injection.
Store the drug frozen at a temperature of -20 to -10°C.
Note
Two to three hours prior to use, thaw the vial in the leaded container, at room temperature.
Discard the unused portion of the drug after 4-6 hours if kept at room temperature.
In conformance with USP recommendations, Iodine 131 preparations should not be used after
the expiration date stated on the label.
NDC # 45567-0100-1
Manufactured in the USA by
Pharmalucence, Inc.
10 DeAngelo Drive
Bedford, MA 01730
This radiopharmaceutical is approved for distribution to persons licensed to use radioactive material listed in Section 120.547, Code of Massachusetts Regulation 105, or under equivalent license of the U.S. Nuclear Regulatory Commission or an Agreement State.
RM 2I-006
03/08
| IOBENGUANE SULFATE I 131 INJECTION
iobenguane sulfate i 131 injection injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA020084 | 03/25/1994 | 09/07/2009 |
| Labeler - Pharmalucence, Inc. (139261648) |