FLUCONAZOLE
-
fluconazole injection, solution
Baxter Healthcare Corporation
----------
FLUCONAZOLE INJECTIONin INTRAVIA Plastic Container
DESCRIPTION
Fluconazole, the first of a new subclass of synthetic triazole antifungal agents, is available as a sterile solution for intravenous use in INTRAVIA plastic container.
Fluconazole is designated chemically as 2,4-difluoro-α, α1-bis(1H-1,2,4-triazol-1-ylmethyl) benzyl alcohol with an empirical formula of C13H12F2N6O and molecular weight 306.3. The structural formula is:
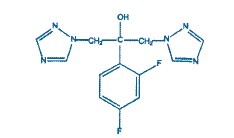
Fluconazole is a white crystalline solid which is slightly soluble in water and saline.
Fluconazole injection is an iso-osmotic, sterile, nonpyrogenic solution of fluconazole in a sodium chloride diluent. Each mL contains 2 mg of fluconazole and 9 mg of sodium chloride. The pH ranges from 4.0 to 8.0. Injection volumes of 100 mL and 200 mL are packaged in INTRAVIA plastic containers.
The flexible container is manufactured from a specially designed multilayer plastic (PL 2408). Solutions in contact with the plastic container leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. The flexible container has a foil overwrap. Water can permeate the plastic into the overwrap, but the amount is insufficient to significantly affect the premixed solution.
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism
The pharmacokinetic properties of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20-50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmax of 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single oral doses of 50-400 mg, fluconazole plasma concentrations and AUC (area under the plasma concentration-time curve) are dose proportional.
Administration of a single oral 150 mg tablet of fluconazole to ten lactating women resulted in a mean Cmax of 2.61 mcg/mL (range: 1.57 to 3.65 mcg/mL).
Steady-state concentrations are reached within 5-10 days following oral doses of 50-400 mg given once daily. Administration of a loading dose (on day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11-12%). Following either single- or multiple-oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the CSF are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue:plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid:plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
| Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 - 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 – 0.7 |
In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of Fluconazole Injection may need to be reduced in patients with impaired renal function. (See DOSAGE AND ADMINISTRATION.) A 3-hour hemodialysis session decreases plasma concentrations by approximately 50%.
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the ACTH-stimulated cortisol response.
Pharmacokinetics in Children
In children, the following pharmacokinetic data {Mean(%cv)} have been reported:
| Age
Studied |
Dose (mg/kg) |
Clearance (mL/min/kg) |
Half-life (Hours) |
Cmax (mcg/mL) |
Vdss (L/kg) |
| 9 Months - 13 years |
Single-Oral 2 mg/kg |
0.40 (38%) N=14 | 25.0 |
2.9 (22%) N=16 | — |
| 9 Months - 13 years |
Single-Oral 8 mg/kg |
0.51 (60%) N=15 | 19.5 |
9.8 (20%) N=15 | — |
| 5 - 15 years |
Multiple IV 2 mg/kg |
0.49 (40%) N=4 | 17.4 |
5.5 (25%) N=5 |
0.722 (36%) N=4 |
| 5 - 15 years |
Multiple IV 4 mg/kg |
0.59 (64%) N=5 | 15.2 |
11.4 (44%) N=6 |
0.729 (33%) N=5 |
| 5 - 15 years |
Multiple IV 8 mg/kg | 0.66
(31%) N=7 | 17.6 | 14.1
(22%) N=8 |
1.069 (37%) N=7 |
Clearance corrected for body weight was not affected by age in these studies. Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg.
In premature newborns (gestational age 26 to 29 weeks), the mean (%cv) clearance within 36 hours of birth was 0.180 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg six days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours six days later and 46.6 hours 12 days later.
Pharmacokinetics in Elderly
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmax was 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg٠h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0-24 hr, 22%) and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared with the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life – creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared with normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
Drug Interaction Studies
Oral contraceptives: Oral contraceptives were administered as a single dose both before and after the oral administration of fluconazole 50 mg once daily for 10 days in 10 healthy women. There was no significant difference in ethinyl estradiol or levonorgestrel AUC after the administration of 50 mg of fluconazole. The mean increase in ethinyl estradiol AUC was 6% (range: -47 to 108%) and levonorgestrel AUC increased 17% (range: -33 to 141%).
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: -12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: -11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range 18 - 31%) and 13% (95% C.I. range 8 - 18%), respectively relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Cimetidine: Fluconazole 100 mg was administered as a single oral dose alone and two hours after a single dose of cimetidine 400 mg to six healthy male volunteers. After the administration of cimetidine, there was a significant decrease in fluconazole AUC and Cmax. There was a mean ± SD decrease in fluconazole AUC of 13% ± 11% (range: -3.4 to -31%) and Cmax decreased 19% ± 14% (range:-5 to -40%). However, the administration of cimetidine 600 mg to 900 mg intravenously over a four-hour period (from one hour before to 3 hours after a single oral dose of fluconazole 200 mg) did not affect the bioavailability or pharmacokinetics of fluconazole in 24 healthy male volunteers.
Antacid: Administration of Maalox® (20 mL) to 14 normal male volunteers immediately prior to a single dose of fluconazole 100 mg had no effect on the absorption or elimination of fluconazole.
Hydrochlorothiazide: Concomitant oral administration of 100 mg fluconazole and 50 mg hydrochlorothiazide for 10 days in 13 normal volunteers resulted in a significant increase in fluconazole AUC and Cmax compared to fluconazole given alone. There was a mean ± SD increase in fluconazole AUC and Cmax of 45% ± 31% (range: 19 to 114%) and 43% ± 31% (range: 19 to 122%), respectively. These changes are attributed to a mean ± SD reduction in renal clearance of 30% ± 12% (range: -10 to -50%).
Rifampin: Administration of a single oral 200 mg dose of fluconazole after 15 days of rifampin administered as 600 mg daily in eight healthy male volunteers resulted in a significant decrease in fluconazole AUC and a significant increase in apparent oral clearance of fluconazole. There was a mean ± SD reduction in fluconazole AUC of 23% ± 9% (range: -13 to -42%). Apparent oral clearance of fluconazole increased 32% ± 17% (range: 16 to 72%). Fluconazole half-life decreased from 33.4 ± 4.4 hours to 26.8 ± 3.9 hours. (See PRECAUTIONS.)
Warfarin: There was a significant increase in prothrombin time response (area under the prothrombin time-time curve) following a single dose of warfarin (15 mg) administered to 13 normal male volunteers following oral fluconazole 200 mg administered daily for 14 days as compared to the administration of warfarin alone. There was a mean ± SD increase in the prothrombin time response (area under the prothrombin time-time curve) of 7% ± 4% (range: -2 to 13%). (See PRECAUTIONS.) Mean is based on data from 12 subjects as one of 13 subjects experienced a 2-fold increase in his prothrombin time response.
Phenytoin: Phenytoin AUC was determined after 4 days of phenytoin dosing (200 mg daily, orally for 3 days followed by 250 mg intravenously for one dose) both with and without the administration of fluconazole (oral fluconazole 200 mg daily for 16 days) in 10 normal male volunteers. There was a significant increase in phenytoin AUC. The mean ± SD increase in phenytoin AUC was 88% ± 68% (range: 16 to 247%). The absolute magnitude of this interaction is unknown because of the intrinsically nonlinear disposition of phenytoin. (See PRECAUTIONS.)
Cyclosporine: Cyclosporine AUC and Cmax were determined before and after the administration of fluconazole 200 mg daily for 14 days in eight renal transplant patients who had been on cyclosporine therapy for at least 6 months and on a stable cyclosporine dose for at least 6 weeks. There was a significant increase in cyclosporine AUC, Cmax, Cmin (24-hour concentration), and a significant reduction in apparent oral clearance following the administration of fluconazole. The mean ± SD increase in AUC was 92% ± 43% (range: 18 to 147%). The Cmax increased 60% ± 48% (range: -5 to 133%). The Cmin increased 157% ± 96% (range: 33 to 360%). The apparent oral clearance decreased 45% ± 15% (range: -15 to -60%). (See PRECAUTIONS.)
Zidovudine: Plasma zidovudine concentrations were determined on two occasions (before and following fluconazole 200 mg daily for 15 days) in 13 volunteers with AIDS or ARC who were on a stable zidovudine dose for at least two weeks. There was a significant increase in zidovudine AUC following the administration of fluconazole. The mean ± SD increase in AUC was 20% ± 32% (range: -27 to 104%). The metabolite, GZDV, to parent drug ratio significantly decreased after the administration of fluconazole, from 7.6 ± 3.6 to 5.7 ± 2.2.
Theophylline: The pharmacokinetics of theophylline were determined from a single intravenous dose of aminophylline (6 mg/kg) before and after the oral administration of fluconazole 200 mg daily for 14 days in 16 normal male volunteers. There were significant increases in theophylline AUC, Cmax, and half-life with a corresponding decrease in clearance. The mean ± SD theophylline AUC increased 21% ± 16% (range: -5 to 48%). The Cmax increased 13% ± 17% (range: -13 to 40%). Theophylline clearance decreased 16% ± 11% (range: -32 to 5%). The half-life of theophylline increased from 6.6 ± 1.7 hours to 7.9 ± 1.5 hours. (See PRECAUTIONS.)
Terfenadine: Six healthy volunteers received terfenadine 60 mg BID for 15 days. Fluconazole 200 mg was administered daily from days 9 through 15. Fluconazole did not affect terfenadine plasma concentrations. Terfenadine acid metabolite AUC increased 36% ± 36% (range: 7 to 102%) from day 8 to day 15 with the concomitant administration of fluconazole. There was no change in cardiac repolarization as measured by Holter QTc intervals. Another study at a 400-mg and 800-mg daily dose of fluconazole demonstrated that fluconazole taken in doses of 400 mg per day or greater significantly increases plasma levels of terfenadine when taken concomitantly. (See CONTRAINDICATIONS and PRECAUTIONS.)
Oral hypoglycemics: The effects of fluconazole on the pharmacokinetics of the sulfonylurea oral hypoglycemic agents tolbutamide, glipizide, and glyburide were evaluated in three placebo-controlled studies in normal volunteers. All subjects received the sulfonylurea alone as a single dose and again as a single dose following the administration of fluconazole 100 mg daily for 7 days. In these three studies 22/46 (47.8%) of fluconazole treated patients and 9/22 (40.1%) of placebo treated patients experienced symptoms consistent with hypoglycemia. (See PRECAUTIONS.)
- Tolbutamide: In 13 normal male volunteers, there was significant increase in tolbutamide (500 mg single dose) AUC and Cmax following the administration of fluconazole. There was a mean ± SD increase in tolbutamide AUC of 26% ± 9% (range: 12 to 39%). Tolbutamide Cmax increased 11% ± 9% (range: -6 to 27%). (See PRECAUTIONS.)
- Glipizide: The AUC and Cmax of glipizide (2.5 mg single dose) were significantly increased following the administration of fluconazole in 13 normal male volunteers. There was a mean ± SD increase in AUC of 49% ± 13% (range: 27 to 73%) and an increase in Cmax of 19% ± 23% (range: -11 to 79%). (See PRECAUTIONS.)
- Glyburide: The AUC and Cmax of glyburide (5 mg single dose) were significantly increased following the administration of fluconazole in 20 normal male volunteers. There was a mean ± SD increase in AUC of 44% ± 29% (range: -13 to 115%) and Cmax increased 19% ± 19% (range: -23 to 62%). Five subjects required oral glucose following the ingestion of glyburide after 7 days of fluconazole administration. (See PRECAUTIONS.)
Rifabutin: There have been published reports that an interaction exists when fluconazole is administered concomitantly with rifabutin, leading to increased serum levels of rifabutin. (See PRECAUTIONS.)
Tacrolimus: There have been published reports that an interaction exists when fluconazole is administered concomitantly with tacrolimus, leading to increased serum levels of tacrolimus. (See PRECAUTIONS.)
Cisapride: A placebo-controlled, randomized, multiple-dose study examined the potential interaction of fluconazole with cisapride. Two groups of 10 normal subjects were administered fluconazole 200 mg daily or placebo. Cisapride 20 mg four times daily was started after 7 days of fluconazole or placebo dosing. Following a single dose of fluconazole, there was a 101% increase in the cisapride AUC and a 91% increase in the cisapride Cmax. Following multiple doses of fluconazole, there was a 192% increase in the cisapride AUC and a 154% increase in the cisapride Cmax. Fluconazole significantly increased the QTc interval in subjects receiving cisapride 20 mg four times daily for 5 days. (See CONTRAINDICATIONS and PRECAUTIONS.)
Midazolam: The effect of fluconazole on the pharmacokinetics and pharmacodynamics of midazolam was examined in a randomized, cross-over study in 12 volunteers. In the study, subjects ingested placebo or 400 mg fluconazole on Day 1 followed by 200 mg daily from Day 2 to Day 6. In addition, a 7.5 mg dose of midazolam was orally ingested on the first day, 0.05 mg/kg was administered intravenously on the fourth day, and 7.5 mg orally on the sixth day. Fluconazole reduced the clearance of IV midazolam by 51%. On the first day of dosing, fluconazole increased the midazolam AUC and Cmax by 259% and 150%, respectively. On the sixth day of dosing, fluconazole increased the midazolam AUC and Cmax by 259% and 74%, respectively. The psychomotor effects of midazolam were significantly increased after oral administration of midazolam but not significantly affected following intravenous midazolam.
A second randomized, double-dummy, placebo-controlled, cross-over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmax of midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmax by 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmax by 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See PRECAUTIONS.)
Azithromycin: An open-label, randomized, three-way crossover study in 18 healthy subjects assessed the effect of a single 800 mg oral dose of fluconazole on the pharmacokinetics of a single 1200 mg oral dose of azithromycin as well as the effects of azithromycin on the pharmacokinetics of fluconazole. There was no significant pharmacokinetic interaction between fluconazole and azithromycin.
Microbiology
Mechanism of Action
Fluconazole is a highly selective inhibitor of fungal cytochrome P-450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl- sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Activity In Vitro and In Clinical Infections
Fluconazole has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections.
Candida albicans
Candida glabrata (Many strains are intermediately susceptible)1
Candida parapsilosis
Candida tropicalis
Cryptococcus neoformans
The following in vitro data are available, but their clinical significance is unknown.
Fluconazole exhibits in vitro minimum inhibitory concentrations (MIC values) of 8 mcg/mL or less against most (≥90%) strains of the following microorganisms, however, the safety and effectiveness of fluconazole in treating clinical infections due to these microorganisms have not been established in adequate and well controlled trials.
Candida dubliniensis
Candida guilliermondii
Candida kefyr
Candida lusitaniae
Candida krusei should be considered to be resistant to fluconazole. Resistance in C. krusei appears to be mediated by reduced sensitivity of the target enzyme to inhibition by the agent.
There have been reports of cases of superinfection with Candida species other than C. albicans, which are often inherently not susceptible to fluconazole (e.g., Candida krusei). Such cases may require alternative antifungal therapy.
- 1
-
In a majority of the studies, fluconazole MIC90 values against C. glabrata were above the susceptible breakpoint (≥16 mcg/mL). Resistance in Candida glabrata usually includes upregulation of CDR genes resulting in resistance to multiple azoles. For an isolate where the MIC is categorized as intermediate (16 to 32 mcg/mL, see Table 1), the highest dose is recommended (see DOSAGE AND ADMINISTRATION). For resistant isolates alternative therapy is recommended.
Susceptibility Testing Methods
Cryptococcus neoformans and filamentous fungi:
No interpretive criteria have been established for Cryptococcus neoformans and filamentous fungi.
Candida species:
Broth Dilution Techniques:
Quantitative methods are used to determine antifungal minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of Candida spp. to antifungal agents. MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (broth)1 with standardized inoculum concentrations of fluconazole powder. The MIC values should be interpreted according to the criteria provided in Table 1.
Diffusion Techniques:
Qualitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of Candida spp. to an antifungal agent. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 25 mcg of fluconazole to test the susceptibility of yeasts to fluconazole. Disk diffusion interpretive criteria are also provided in Table 1.
| Table 1: Susceptibility Interpretive Criteria for Fluconazole | ||||||
| Broth Dilution at 48
hours (MIC in mcg/mL) | Disk Diffusion at 24
hours (Zone Diameters in mm) |
|||||
| Antifungal agent | Susceptible (S) | Intermediate (I)* | Resistant (R) | Susceptible (S) | Intermediate (I)* | Resistant (R) |
| Fluconazole† | ≤8.0 | 16-32 | ≥64 | ≥19 | 15 – 18 | ≤14 |
The susceptible category implies that isolates are inhibited by the usually achievable concentrations of antifungal agent tested when the recommended dosage is used. The intermediate category implies that an infection due to the isolate may be appropriately treated in body sites where the drugs are physiologically concentrated or when a high dosage of drug is used. The resistant category implies that isolates are not inhibited by the usually achievable concentrations of the agent with normal dosage schedules and clinical efficacy of the agent against the isolate has not been reliably shown in treatment studies.
Quality Control
Standardized susceptibility test procedures require the use of quality control organisms to control the technical aspects of the test procedures. Standardized fluconazole powder and 25 mcg disks should provide the following range of values noted in Table 2. NOTE: Quality control microorganisms are specific strains of organisms with intrinsic biological properties relating to resistance mechanisms and their genetic expression within fungi; the specific strains used for microbiological control are not clinically significant.
|
|||
| Table 2. Acceptable Quality Control Ranges for Fluconazole to be Used in Validation of Susceptibility Test Results | |||
| QC Strain | Macrodilution (MIC in mcg/mL) @48 hours | Microdilution (MIC in mcg/mL) @ 48 hours | Disk Diffusion (Zone Diameter in mm) @ 24 hours |
| Candida parapsilosis ATCC 22019 | 2.0-8.0 | 1.0-4.0 | 22-33 |
| Candida krusei ATCC 6258 | 16-64 | 16-128 | ---* |
| Candida albicans ATCC 90028 | ---* | ---* | 28-39 |
| Candida tropicalis ATCC 750 | ---* | ---* | 26-37 |
Activity In Vivo
Fungistatic activity has also been demonstrated in normal and immunocompromised animal models for systemic and intracranial fungal infections due to Cryptococcus neoformans and for systemic infections due to Candida albicans.
In common with other azole antifungal agents, most fungi show a higher apparent sensitivity to fluconazole in vivo than in vitro. Fluconazole administered orally and/or intravenously was active in a variety of animal models of fungal infection using standard laboratory strains of fungi. Activity has been demonstrated against fungal infections caused by Aspergillus flavus and Aspergillus fumigatus in normal mice. Fluconazole has also been shown to be active in animal models of endemic mycoses, including one model of Blastomyces dermatitidis pulmonary infections in normal mice; one model of Coccidioides immitis intracranial infections in normal mice; and several models of Histoplasma capsulatum pulmonary infection in normal and immunosuppressed mice. The clinical significance of results obtained in these studies is unknown.
Concurrent administration of fluconazole and amphotericin B in infected normal and immunosuppressed mice showed the following results: a small additive antifungal effect in systemic infection with C. albicans, no interaction in intracranial infection with Cryptococcus neoformans, and antagonism of the two drugs in systemic infection with A. fumigatus. The clinical significance of results obtained in these studies is unknown.
Drug Resistance
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (ERG11) encoding for the target enzyme lead to an altered target with decreased affinity for azoles. Overexpression of ERG11 results in the production of high concentrations of the target enzyme, creating the need for higher intracellular drug concentrations to inhibit all of the enzyme molecules in the cell.
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by MDR genes) and those of the ATP-binding cassette superfamily (encoded by CDR genes). Upregulation of the MDR gene leads to fluconazole resistance, whereas, upregulation of CDR genes may lead to resistance to multiple azoles.
Resistance in Candida glabrata usually includes upregulation of CDR genes resulting in resistance to multiple azoles. For an isolate where the MIC is categorized as Intermediate (16 to 32 mcg/mL), the highest fluconazole dose is recommended.
Candida krusei should be considered to be resistant to fluconazole. Resistance in C. krusei appears to be mediated by reduced sensitivity of the target enzyme to inhibition by the agent.
There have been reports of cases of superinfection with Candida species other than C. albicans, which are often inherently not susceptible to fluconazole (e.g., Candida krusei). Such cases may require alternative antifungal therapy.
INDICATIONS AND USAGE
Fluconazole Injection is indicated for the treatment of:
- Oropharyngeal and esophageal candidiasis. In open noncomparative studies of relatively small numbers of patients, fluconazole was also effective for the treatment of Candida urinary tract infections, peritonitis, and systemic Candida infections including candidemia, disseminated candidiasis, and pneumonia.
- Cryptococcal meningitis. Before prescribing Fluconazole Injection for AIDS patients with cryptococcal meningitis, please see CLINICAL STUDIES section. Studies comparing fluconazole to amphotericin B in non-HIV infected patients have not been conducted.
Prophylaxis - Fluconazole Injection is also indicated to decrease the incidence of candidiasis in patients undergoing bone marrow transplantation who receive cytotoxic chemotherapy and/or radiation therapy.
Specimens for fungal culture and other relevant laboratory studies (serology, histopathology) should be obtained prior to therapy to isolate and identify causative organisms. Therapy may be instituted before the results of the cultures and other laboratory studies are known; however, once these results become available, anti-infective therapy should be adjusted accordingly.
CLINICAL STUDIES
Cryptococcal meningitis: In a multicenter study comparing fluconazole (200 mg/day) to amphotericin B (0.3 mg/kg/day) for treatment of cryptococcal meningitis in patients with AIDS, a multivariate analysis revealed three pretreatment factors that predicted death during the course of therapy: abnormal mental status, cerebrospinal fluid cryptococcal antigen titer greater than 1:1024, and cerebrospinal fluid white blood cell count of less than 20 cells/mm 3. Mortality among high risk patients was 33% and 40% for amphotericin B and fluconazole patients, respectively (p=0.58), with overall deaths 14% (9 of 63 subjects) and 18% (24 of 131 subjects) for the 2 arms of the study (p=0.48). Optimal doses and regimens for patients with acute cryptococcal meningitis and at high risk for treatment failure remain to be determined. (Saag, et al. N Engl J Med 1992; 326:83-9.)
Pediatric Studies
Oropharyngeal candidiasis: An open-label, comparative study of the efficacy and safety of fluconazole (2-3 mg/kg/day) and oral nystatin (400,000 I.U. 4 times daily) in immunocompromised children with oropharyngeal candidiasis was conducted. Clinical and mycological response rates were higher in the children treated with fluconazole.
Clinical cure at the end of treatment was reported for 86% of fluconazole treated patients compared to 46% of nystatin treated patients. Mycologically, 76% of fluconazole treated patients had the infecting organism eradicated compared to 11% for nystatin treated patients.
|
||
| Fluconazole | Nystatin | |
| Enrolled | 96 | 90 |
| Clinical Cure | 76/88 (86%) | 36/78 (46%) |
| Mycological eradication* | 55/72 (76%) | 6/54 (11%) |
The proportion of patients with clinical relapse 2 weeks after the end of treatment was 14% for subjects receiving fluconazole and 16% for subjects receiving nystatin. At 4 weeks after the end of treatment the percentages of patients with clinical relapse were 22% for fluconazole and 23% for nystatin.
CONTRAINDICATIONS
Fluconazole Injection is contraindicated in patients who have shown hypersensitivity to fluconazole or to any of its excipients. There is no information regarding cross-hypersensitivity between fluconazole and other azole antifungal agents. Caution should be used in prescribing Fluconazole Injection to patients with hypersensitivity to other azoles. Coadministration of terfenadine is contraindicated in patients receiving Fluconazole Injection at multiple doses of 400 mg or higher based upon results of a multiple dose interaction study. Coadministration of cisapride is contraindicated in patients receiving Fluconazole Injection. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies and PRECAUTIONS.)
WARNINGS
- Hepatic injury: Fluconazole has been associated with rare cases of serious hepatic toxicity, including fatalities primarily in patients with serious underlying medical conditions. In cases of fluconazole-associated hepatotoxicity, no obvious relationship to total daily dose, duration of therapy, sex or age of the patient has been observed. Fluconazole hepatotoxicity has usually, but not always, been reversible on discontinuation of therapy. Patients who develop abnormal liver function tests during Fluconazole Injection therapy should be monitored for the development of more severe hepatic injury. Fluconazole Injection should be discontinued if clinical signs and symptoms consistent with liver disease develop that may be attributable to fluconazole.
- Anaphylaxis: In rare cases, anaphylaxis has been reported.
- Dermatologic: Patients have rarely developed exfoliative skin disorders during treatment with fluconazole. In patients with serious underlying diseases (predominantly AIDS and malignancy), these have rarely resulted in a fatal outcome. Patients who develop rashes during treatment with Fluconazole Injection should be monitored closely and the drug discontinued if lesions progress.
PRECAUTIONS
General
Some azoles, including fluconazole, have been associated with prolongation of the QT interval on the electrocardiogram. During post-marketing surveillance, there have been rare cases of QT prolongation and torsade de pointes in patients taking fluconazole. Most of these reports involved seriously ill patients with multiple confounding risk factors, such as structural heart disease, electrolyte abnormalities and concomitant medications that may have been contributory.
Fluconazole Injection should be administered with caution to patients with these potentially proarrhythmic conditions.
Drug Interactions
(See CLINICAL PHARMACOLOGY: Drug Interaction Studies and CONTRAINDICATIONS.) Clinically or potentially significant drug interactions between fluconazole and the following agents/classes have been observed. These are described in greater detail below:
Oral hypoglycemics
Coumarin-type anticoagulants
Phenytoin
Cyclosporine
Rifampin
Theophylline
Terfenadine
Cisapride
Astemizole
Rifabutin
Tacrolimus
Short-acting benzodiazepines
Oral hypoglycemics: Clinically significant hypoglycemia may be precipitated by the use of fluconazole with oral hypoglycemic agents; one fatality has been reported from hypoglycemia in association with combined fluconazole and glyburide use. Fluconazole reduces the metabolism of tolbutamide, glyburide, and glipizide and increases the plasma concentration of these agents. When Fluconazole Injection is used concomitantly with these or other sulfonylurea oral hypoglycemic agents, blood glucose concentrations should be carefully monitored and the dose of the sulfonylurea should be adjusted as necessary. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Coumarin-type anticoagulants: Prothrombin time may be increased in patients receiving concomitant fluconazole and coumarin-type anticoagulants. In post-marketing experience, as with other azole antifungals, bleeding events (bruising, epistaxis, gastrointestinal bleeding, hematuria, and melena) have been reported in association with increases in prothrombin time in patients receiving fluconazole concurrently with warfarin. Careful monitoring of prothrombin time in patients receiving Fluconazole Injection and coumarin-type anticoagulants is recommended. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Phenytoin: Fluconazole increases the plasma concentrations of phenytoin. Careful monitoring of phenytoin concentrations in patients receiving Fluconazole Injection and phenytoin is recommended. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Cyclosporine: Fluconazole may significantly increase cyclosporine levels in renal transplant patients with or without renal impairment. Careful monitoring of cyclosporine concentrations and serum creatinine is recommended in patients receiving Fluconazole Injection and cyclosporine. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Rifampin: Rifampin enhances the metabolism of concurrently administered fluconazole. Depending on clinical circumstances, consideration should be given to increasing the dose of Fluconazole Injection when it is administered with rifampin. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Theophylline: Fluconazole increases the serum concentrations of theophylline. Careful monitoring of serum theophylline concentrations in patients receiving Fluconazole Injection and theophylline is recommended. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Terfenadine: Because of the occurrence of serious cardiac dysrhythmias secondary to prolongation of the QTc interval in patients receiving azole antifungals in conjunction with terfenadine, interaction studies have been performed. One study at a 200-mg daily dose of fluconazole failed to demonstrate a prolongation in QTc interval. Another study at a 400-mg and 800-mg daily dose of fluconazole demonstrated that fluconazole taken in doses of 400 mg per day or greater significantly increases plasma levels of terfenadine when taken concomitantly. The combined use of Fluconazole Injection at doses of 400 mg or greater with terfenadine is contraindicated. (See CONTRAINDICATIONS and CLINICAL PHARMACOLOGY: Drug Interaction Studies.) The coadministration of Fluconazole Injection at doses lower than 400 mg/day with terfenadine should be carefully monitored.
Cisapride: There have been reports of cardiac events, including torsade de pointes in patients to whom fluconazole and cisapride were coadministered. A controlled study found that concomitant fluconazole 200 mg once daily and cisapride 20 mg four times a day yielded a significant increase in cisapride plasma levels and prolongation of QTc interval. The combined use of Fluconazole Injection with cisapride is contraindicated. (See CONTRAINDICATIONS and CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Astemizole: The use of fluconazole in patients concurrently taking astemizole or other drugs metabolized by the cytochrome P450 system may be associated with elevations in serum levels of these drugs. In the absence of definitive information, caution should be used when coadministering Fluconazole Injection. Patients should be carefully monitored.
Rifabutin: There have been reports of uveitis in patients to whom fluconazole and rifabutin were coadministered. Patients receiving rifabutin and Fluconazole Injection concomitantly should be carefully monitored. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Tacrolimus: There have been reports of nephrotoxicity in patients to whom fluconazole and tacrolimus were coadministered. Patients receiving tacrolimus and Fluconazole Injection concomitantly should be carefully monitored. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Short-acting Benzodiazepines: Following oral administration of midazolam, fluconazole resulted in substantial increases in midazolam concentrations and psychomotor effects. This effect on midazolam appears to be more pronounced following oral administration of fluconazole than with fluconazole administered intravenously. If short-acting benzodiazepines, which are metabolized by the cytochrome P450 system, are concomitantly administered with Fluconazole Injection, consideration should be given to decreasing the benzodiazepine dosage, and the patients should be appropriately monitored. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.)
Fluconazole tablets coadministered with ethinyl estradiol- and levonorgestrel-containing oral contraceptives produced an overall mean increase in ethinyl estradiol and levonorgestrel levels; however, in some patients there were decreases up to 47% and 33% of ethinyl estradiol and levonorgestrel levels. (See CLINICAL PHARMACOLOGY: Drug Interaction Studies.) The data presently available indicate that the decreases in some individual ethinyl estradiol and levonorgestrel AUC values with fluconazole treatment are likely the result of random variation. While there is evidence that fluconazole can inhibit the metabolism of ethinyl estradiol and levonorgestrel, there is no evidence that fluconazole is a net inducer of ethinyl estradiol or levonorgestrel metabolism. The clinical significance of these effects is presently unknown.
Physicians should be aware that interaction studies with medications other than those listed in the CLINICAL PHARMACOLOGY section have not been conducted, but such interactions may occur.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Fluconazole showed no evidence of carcinogenic potential in mice and rats treated orally for 24 months at doses of 2.5, 5 or 10 mg/kg/day (approximately 2-7x the recommended human dose). Male rats treated with 5 and 10 mg/kg/day had an increased incidence of hepatocellular adenomas.
Fluconazole, with or without metabolic activation, was negative in tests for mutagenicity in 4 strains of S. typhimurium, and in the mouse lymphoma L5178Y system. Cytogenetic studies in vivo (murine bone marrow cells, following oral administration of fluconazole) and in vitro (human lymphocytes exposed to fluconazole at 1000 mcg/mL) showed no evidence of chromosomal mutations.
Fluconazole did not affect the fertility of male or female rats treated orally with daily doses of 5, 10 or 20 mg/kg or with parenteral doses of 5, 25 or 75 mg/kg, although the onset of parturition was slightly delayed at 20 mg/kg PO. In an intravenous perinatal study in rats at 5, 20 and 40 mg/kg, dystocia and prolongation of parturition were observed in a few dams at 20 mg/kg (approximately 5-15x the recommended human dose) and 40 mg/kg, but not at 5 mg/kg. The disturbances in parturition were reflected by a slight increase in the number of still-born pups and decrease of neonatal survival at these dose levels. The effects on parturition in rats are consistent with the species specific estrogen-lowering property produced by high doses of fluconazole. Such a hormone change has not been observed in women treated with fluconazole. (See CLINICAL PHARMACOLOGY.)
Pregnancy
Teratogenic Effects
Pregnancy Category C
Fluconazole was administered orally to pregnant rabbits during organogenesis in two studies, at 5, 10 and 20 mg/kg and at 5, 25, and 75 mg/kg, respectively. Maternal weight gain was impaired at all dose levels, and abortions occurred at 75 mg/kg (approximately 20-60x the recommended human dose); no adverse fetal effects were detected. In several studies in which pregnant rats were treated orally with fluconazole during organogenesis, maternal weight gain was impaired and placental weights were increased at 25 mg/kg. There were no fetal effects at 5 or 10 mg/kg; increases in fetal anatomical variants (supernumerary ribs, renal pelvis dilation) and delays in ossification were observed at 25 and 50 mg/kg and higher doses. At doses ranging from 80 mg/kg (approximately 20-60x the recommended human dose) to 320 mg/kg embryolethality in rats was increased and fetal abnormalities included wavy ribs, cleft palate and abnormal cranio-facial ossification. These effects are consistent with the inhibition of estrogen synthesis in rats and may be a result of known effects of lowered estrogen on pregnancy, organogenesis and parturition.
There are no adequate and well controlled studies in pregnant women. There have been reports of multiple congenital abnormalities in infants whose mothers were being treated for 3 or more months with high dose (400-800 mg/day) fluconazole therapy for coccidioidomycosis (an unindicated use). The relationship between Fluconazole Injection use and these events is unclear. Fluconazole should be used in pregnancy only if the potential benefit justifies the possible risk to the fetus.
Nursing Mothers
Fluconazole is secreted in human milk at concentrations similar to plasma. Therefore, the use of Fluconazole Injection in nursing mothers is not recommended.
Pediatric Use
An open-label, randomized, controlled trial has shown fluconazole to be effective in the treatment of oropharyngeal candidiasis in children 6 months to 13 years of age. (See CLINICAL STUDIES.)
The use of fluconazole in children with cryptococcal meningitis, Candida esophagitis, or systemic Candida infections is supported by the efficacy shown for these indications in adults and by the results from several small noncomparative pediatric clinical studies. In addition, pharmacokinetic studies in children (see CLINICAL PHARMACOLOGY) have established a dose proportionality between children and adults. (See DOSAGE AND ADMINISTRATION.)
In a noncomparative study of children with serious systemic fungal infections, most of which were candidemia, the effectiveness of fluconazole was similar to that reported for the treatment of candidemia in adults. Of 17 subjects with culture-confirmed candidemia, 11 of 14 (79%) with baseline symptoms (3 were asymptomatic) had a clinical cure; 13/15 (87%) of evaluable patients had a mycologic cure at the end of treatment but two of these patients relapsed at 10 and 18 days, respectively, following cessation of therapy.
The efficacy of fluconazole for the suppression of cryptococcal meningitis was successful in 4 of 5 children treated in a compassionate-use study of fluconazole for the treatment of life-threatening or serious mycosis. There is no information regarding the efficacy of fluconazole for primary treatment of cryptococcal meningitis in children.
The safety profile of fluconazole in children has been studied in 577 children ages 1 day to 17 years who received doses ranging from 1 to 15 mg/kg/day for 1 to 1,616 days. (See ADVERSE REACTIONS.)
Efficacy of fluconazole has not been established in infants less than 6 months of age. (See CLINICAL PHARMACOLOGY.) A small number of patients (29) ranging in age from 1 day to 6 months have been treated safely with fluconazole.
Geriatric Use
In non-AIDS patients, side effects possibly related to fluconazole treatment were reported in fewer patients aged 65 and older (9%, n=339) than for younger patients (14%, n=2240). However, there was no consistent difference between the older and younger patients with respect to individual side effects. Of the most frequently reported (>1%) side effects, rash, vomiting and diarrhea occurred in greater proportions of older patients. Similar proportions of older patients (2.4%) and younger patients (1.5%) discontinued fluconazole therapy because of side effects. In post-marketing experience, spontaneous reports of anemia and acute renal failure were more frequent among patients 65 years of age or older than in those between 12 and 65 years of age. Because of the voluntary nature of the reports and the natural increase in the incidence of anemia and renal failure in the elderly, it is however not possible to establish a casual relationship to drug exposure.
Controlled clinical trials of fluconazole did not include sufficient numbers of patients aged 65 and older to evaluate whether they respond differently from younger patients in each indication. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Fluconazole is primarily cleared by renal excretion as unchanged drug. Because elderly patients are more likely to have decreased renal function, care should be taken to adjust dose based on creatinine clearance. It may be useful to monitor renal function. (See CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION.)
ADVERSE REACTIONS
In Patients Receiving Multiple Doses:
Sixteen percent of over 4000 patients treated with fluconazole in clinical trials of 7 days or more experienced adverse events. Treatment was discontinued in 1.5% of patients due to adverse clinical events and in 1.3% of patients due to laboratory test abnormalities.
Clinical adverse events were reported more frequently in HIV infected patients (21%) than in non-HIV infected patients (13%); however, the patterns in HIV infected and non-HIV infected patients were similar. The proportions of patients discontinuing therapy due to clinical adverse events were similar in the two groups (1.5%).
The following treatment-related clinical adverse events occurred at an incidence of 1% or greater in 4048 patients receiving fluconazole for 7 or more days in clinical trials: nausea 3.7%, headache 1.9%, skin rash 1.8%, vomiting 1.7%, abdominal pain 1.7%, and diarrhea 1.5%.
Hepatobiliary: In combined clinical trials and marketing experience, there have been rare cases of serious hepatic reactions during treatment with fluconazole. (See WARNINGS.) The spectrum of these hepatic reactions has ranged from mild transient elevations in transaminases to clinical hepatitis, cholestasis and fulminant hepatic failure, including fatalities. Instances of fatal hepatic reactions were noted to occur primarily in patients with serious underlying medical conditions (predominantly AIDS or malignancy) and often while taking multiple concomitant medications. Transient hepatic reactions, including hepatitis and jaundice, have occurred among patients with no other identifiable risk factors. In each of these cases, liver function returned to baseline on discontinuation of fluconazole.
In two comparative trials evaluating the efficacy of fluconazole for the suppression of relapse of cryptococcal meningitis, a statistically significant increase was observed in median AST (SGOT) levels from a baseline value of 30 IU/L to 41 IU/L in one trial and 34 IU/L to 66 IU/L in the other. The overall rate of serum transaminase elevations of more than 8 times the upper limit of normal was approximately 1% in fluconazole-treated patients in clinical trials. These elevations occurred in patients with severe underlying disease, predominantly AIDS or malignancies, most of whom were receiving multiple concomitant medications, including many known to be hepatotoxic. The incidence of abnormally elevated serum transaminases was greater in patients taking fluconazole concomitantly with one or more of the following medications: rifampin, phenytoin, isoniazid, valproic acid, or oral sulfonylurea hypoglycemic agents.
Post-Marketing Experience
In addition, the following adverse events have occurred during postmarketing experience.
Immunologic: In rare cases, anaphylaxis (including angioedema, face edema and pruritus) has been reported.
Cardiovascular: QT prolongation, torsade de pointes. (See PRECAUTIONS.)
Central Nervous System: Seizures, dizziness.
Dermatologic: Exfoliative skin disorders including Stevens-Johnson syndrome and toxic epidermal necrolysis (see WARNINGS), alopecia.
Hematopoietic and Lymphatic: Leukopenia, including neutropenia and agranulocytosis, thrombocytopenia.
Metabolic: Hypercholesterolemia, hypertriglyceridemia, hypokalemia.
Gastrointestinal: Dyspepsia, vomiting.
Other senses: Taste perversion.
Adverse Reactions in Children:
In Phase II/III clinical trials conducted in the United States and in Europe, 577 pediatric patients, ages 1 day to 17 years were treated with fluconazole at doses up to 15 mg/kg/day for up to 1,616 days. Thirteen percent of children experienced treatment related adverse events. The most commonly reported events were vomiting (5%), abdominal pain (3%), nausea (2%), and diarrhea (2%). Treatment was discontinued in 2.3% of patients due to adverse clinical events and in 1.4% of patients due to laboratory test abnormalities. The majority of treatment-related laboratory abnormalities were elevations of transaminases or alkaline phosphatase.
| Percentage of Patients With Treatment-Related Side Effects | ||
| Fluconazole
(N=577) | Comparative Agents
(N=451) |
|
| With any side effect | 13.0 | 9.3 |
| Vomiting | 5.4 | 5.1 |
| Abdominal pain | 2.8 | 1.6 |
| Nausea | 2.3 | 1.6 |
| Diarrhea | 2.1 | 2.2 |
OVERDOSAGE
There have been reports of overdosage with fluconazole. A 42-year-old patient infected with human immunodeficiency virus developed hallucinations and exhibited paranoid behavior after reportedly ingesting 8200 mg of fluconazole. The patient was admitted to the hospital, and his condition resolved within 48 hours.
In the event of overdose, symptomatic treatment (with supportive measures and gastric lavage if clinically indicated) should be instituted.
Fluconazole is largely excreted in urine. A three-hour hemodialysis session decreases plasma levels by approximately 50%.
In mice and rats receiving very high doses of fluconazole, clinical effects in both species included decreased motility and respiration, ptosis, lacrimation, salivation, urinary incontinence, loss of righting reflex and cyanosis; death was sometimes preceded by clonic convulsions.
DOSAGE AND ADMINISTRATION
Dosage and Administration in Adults:
SINCE ORAL ABSORPTION IS RAPID AND ALMOST COMPLETE, THE DAILY DOSE OF FLUCONAZOLE IS THE SAME FOR ORAL AND INTRAVENOUS ADMINISTRATION. In general, a loading dose of twice the daily dose is recommended on the first day of therapy to result in plasma concentrations close to steady-state by the second day of therapy.
The daily dose of Fluconazole Injection for the treatment of infections should be based on the infecting organism and the patient’s response to therapy. Treatment should be continued until clinical parameters or laboratory tests indicate that active fungal infection has subsided. An inadequate period of treatment may lead to recurrence of active infection. Patients with AIDS and cryptococcal meningitis or recurrent oropharyngeal candidiasis usually require maintenance therapy to prevent relapse.
Oropharyngeal candidiasis: The recommended dosage of Fluconazole Injection for oropharyngeal candidiasis is 200 mg on the first day, followed by 100 mg once daily. Clinical evidence of oropharyngeal candidiasis generally resolves within several days, but treatment should be continued for at least 2 weeks to decrease the likelihood of relapse.
Esophageal candidiasis: The recommended dosage of Fluconazole Injection for esophageal candidiasis is 200 mg on the first day, followed by 100 mg once daily. Doses up to 400 mg/day may be used, based on medical judgment of the patient’s response to therapy. Patients with esophageal candidiasis should be treated for a minimum of three weeks and for at least two weeks following resolution of symptoms.
Systemic Candida infections: For systemic Candida infections including candidemia, disseminated candidiasis, and pneumonia, optimal therapeutic dosage and duration of therapy have not been established. In open, noncomparative studies of small numbers of patients, doses of up to 400 mg daily have been used.
Urinary tract infections and peritonitis: For the treatment of Candida urinary tract infections and peritonitis, daily doses of 50-200 mg have been used in open, noncomparative studies of small numbers of patients.
Cryptococcal meningitis: The recommended dosage for treatment of acute cryptococcal meningitis is 400 mg on the first day, followed by 200 mg once daily. A dosage of 400 mg once daily may be used, based on medical judgment of the patient’s response to therapy. The recommended duration of treatment for initial therapy of cryptococcal meningitis is 10-12 weeks after the cerebrospinal fluid becomes culture negative. The recommended dosage of Fluconazole Injection for suppression of relapse of cryptococcal meningitis in patients with AIDS is 200 mg once daily.
Prophylaxis in patients undergoing bone marrow transplantation: The recommended Fluconazole Injection daily dosage for the prevention of candidiasis in patients undergoing bone marrow transplantation is 400 mg, once daily. Patients who are anticipated to have severe granulocytopenia (less than 500 neutrophils per cu mm) should start Fluconazole Injection prophylaxis several days before the anticipated onset of neutropenia, and continue for 7 days after the neutrophil count rises above 1000 cells per cu mm.
Dosage and Administration in Children:
The following dose equivalency scheme should generally provide equivalent exposure in pediatric and adult patients:
|
|
| Pediatric Patients | Adults |
| 3 mg/kg | 100 mg |
| 6 mg/kg | 200 mg |
| 12* mg/kg | 400 mg |
Experience with fluconazole in neonates is limited to pharmacokinetic studies in premature newborns. (See CLINICAL PHARMACOLOGY.) Based on the prolonged half-life seen in premature newborns (gestational age 26 to 29 weeks), these children, in the first two weeks of life, should receive the same dosage (mg/kg) as in older children, but administered every 72 hours. After the first two weeks, these children should be dosed once daily. No information regarding fluconazole pharmacokinetics in full-term newborns is available.
Oropharyngeal candidiasis: The recommended dosage of Fluconazole Injection for oropharyngeal candidiasis in children is 6 mg/kg on the first day, followed by 3 mg/kg once daily. Treatment should be administered for at least 2 weeks to decrease the likelihood of relapse.
Esophageal candidiasis: For the treatment of esophageal candidiasis, the recommended dosage of Fluconazole Injection in children is 6 mg/kg on the first day, followed by 3 mg/kg once daily. Doses up to 12 mg/kg/day may be used based on medical judgment of the patient’s response to therapy. Patients with esophageal candidiasis should be treated for a minimum of 3 weeks and for at least 2 weeks following the resolution of symptoms.
Systemic Candida infections: For the treatment of candidemia and disseminated Candida infections, daily doses of 6-12 mg/kg/day have been used in an open, noncomparative study of a small number of children.
Cryptococcal meningitis: For the treatment of acute cryptococcal meningitis, the recommended dosage is 12 mg/kg on the first day, followed by 6 mg/kg once daily. A dosage of 12 mg/kg once daily may be used, based on medical judgment of the patient’s response to therapy. The recommended duration of treatment for initial therapy of cryptococcal meningitis is 10-12 weeks after the cerebrospinal fluid becomes culture negative. For suppression of relapse of cryptococcal meningitis in children with AIDS, the recommended dose of Fluconazole Injection is 6 mg/kg once daily.
Dosage In Patients With Impaired Renal Function:
Fluconazole is cleared primarily by renal excretion as unchanged drug. In patients with impaired renal function who will receive multiple doses of Fluconazole Injection, an initial loading dose of 50 to 400 mg should be given. After the loading dose, the daily dose (according to indication) should be based on the following table:
| Creatinine Clearance (mL/min) | Percent of Recommended Dose |
| >50 | 100% |
| ≤50 (no dialysis) | 50% |
| Regular dialysis | 100% after each dialysis |
These are suggested dose adjustments based on pharmacokinetics following administration of multiple doses. Further adjustment may be needed depending upon clinical condition.
When serum creatinine is the only measure of renal function available, the following formula (based on sex, weight, and age of the patient) should be used to estimate the creatinine clearance in adults:
| Males: | Weight (kg) x (140 - age) |
| 72 x serum creatinine (mg/100 mL) | |
| Females: | 0.85 x above value |
Although the pharmacokinetics of fluconazole has not been studied in children with renal insufficiency, dosage reduction in children with renal insufficiency should parallel that recommended for adults. The following formula may be used to estimate creatinine clearance in children:
| K x | Linear length or height (cm) |
| Serum creatinine (mg/100 mL) |
(Where K=0.55 for children older than 1 year and 0.45 for infants.)
Administration
Fluconazole Injection is administered by intravenous infusion. Fluconazole injection has been used safely for up to fourteen days of intravenous therapy. The intravenous infusion of Fluconazole Injection should be administered at a maximum rate of approximately 200 mg/hour, given as a continuous infusion.
Fluconazole Injection in INTRAVIA plastic containers is intended only for intravenous administration using sterile equipment.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Do not use if the solution is cloudy or precipitated or if the seal is not intact.
Directions for IV Use of Fluconazole Injection in INTRAVIA Plastic Containers
Do not remove unit from overwrap until ready for use. The overwrap is a moisture barrier. The inner bag maintains the sterility of the product.
CAUTION: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
To Open
Tear overwrap down side at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. After removing overwrap, check for minute leaks by squeezing inner bag firmly. If leaks are found, discard solution as sterility may be impaired.
DO NOT ADD SUPPLEMENTARY MEDICATION.
Preparation for Administration:
- Suspend container from eyelet support.
- Remove plastic protector from outlet port at bottom of container.
- Attach administration set. Refer to complete directions accompanying set.
HOW SUPPLIED
Fluconazole Injections: Fluconazole injections for intravenous infusion administration are formulated as sterile iso-osmotic solutions containing 2 mg/mL of fluconazole. They are supplied in INTRAVIA plastic containers containing volumes of 100 mL or 200 mL affording doses of 200 mg and 400 mg of fluconazole, respectively.
Fluconazole Injections in INTRAVIA Plastic Containers:
| 2J1446 | NDC 0338-6046-48 | Fluconazole in Sodium Chloride Diluent | 200 mg/100 mL x 10 |
| 2J1445 | NDC 0338-6045-37 | Fluconazole in Sodium Chloride Diluent | 400 mg/200 mL x 10 |
Storage
Store between 77°F (25°C) and 41°F (5°C). Brief exposure up to 104°F (40°C) does not adversely affect the product. Protect from freezing. Avoid excessive heat.
Rx only
REFERENCES
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Second Edition. CLSI Document M27-A2, 2002 Volume 22, No 15, CLSI, Wayne, PA, August 2002.
- Clinical and Laboratory Standards Institute. Methods for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline. CLSI Document M44-A, 2004 Volume 24, No. 15 CLSI, Wayne, PA, May 2004.
- Pfaller, M.A., Messer, S.A., Boyken, L., Rice, C., Tendolkar, S., Hollis, R.J., and Diekemal, D.J. Use of Fluconazole as a Surrogate Marker To Predict Susceptibility and Resistance to Voriconazole among 13,338 Clinical Isolates of Candida spp. Tested by Clinical and Laboratory Standard Institute-Recommended Broth Microdilution Methods. 2007. Journal of Clinical Microbiology. 45:70-75.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
07-19-57-803
Rev. August 2008
Maalox® is a registered trademark of Aventis Pharmaceuticals Products Inc.
BAXTER and INTRAVIA are trademarks of Baxter International Inc.
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
Container Label
LOT
EXP
200 mL
2J1445 NDC 0338-6045-37
Fluconazole Injection
400 mg/200mL (2mg/mL)*
ISO-OSMOTIC SODIUM CHLORIDE DILUENT
SINGLE-DOSE STERILE
INTRAVIA CONTAINER NONPYROGENIC
*EACH 200 mL CONTAINS 400 mg OF FLUCONAZOLE AND 1.8 g OF SODIUM CHLORIDE USP IN WATER FOR INJECTION USP
OSMOLARITY 315 mOsmol/L (CALC)
pH 5.5 (4.0 TO 8.0)
NO PRESERVATIVE IS ADDED
USUAL DOSAGE INTRAVENOUSLY AS DIRECTED BY A PHYSICIAN SEE PACKAGE INSERT
CAUTIONS CHECK FOR MINUTE LEAKS BY SQUEEZING CONTAINER FIRMLY IF LEAKS ARE FOUND DISCARD CONTAINER AS STERILITY MAY BE IMPAIRED DO NOT ADD SUPPLEMENTARY MEDICATION MUST NOT BE USED IN SERIES CONNECTIONS USE ONLY IF SOLUTION IS CLEAR
RX ONLY
BAXTER
Baxter Healthcare Corporation
Deerfield IL 60015 USA
MADE IN USA
BAXTER AND INTRAVIA ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
Overwrap Label

Do not remove unit from overwrap until ready to use.
Do not use if overwrap has been previously opened or damaged. The overwrap is a moisture barrier.
The inner bag maintains the sterility of the product.
200 mL 2J1445
NDC 0338-6045-37
Fluconazole Injection
400 mg / 200 mL (2mg/mL)*
ISO-OSMOTIC SODIUM CHLORIDE DILUENT
400 mg STERILE NONPYROGENIC
SINGLE DOSE CONTANER
*Each 200 mL contains: 400 mg of Fluconazole and 1.8 g of Sodium Chloride, USP in Water for Injection, USP. Osmolarity 315 mOsmol/L (calc). pH 5.5 (4.0 to 8.0). No preservative is added.
USUAL DOSAGE: Intravenously as directed by a physician. See package insert.
CAUTIONS: After removing the overwrap, check for minute leaks by squeezing container firmly. If leak are found, discard container as sterility may be impaired. Do not add supplementary medication. Must not be used in series connections. Use only if solution is clear.
Recommended Storage: Store between 77°F (25°C) and 41°F (5°C). Brief exposure up to 104°F (40°C) does not adversely affect the product. Protect from freezing. Avoid excessive heat.
Rx Only
Baxter
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in USA
BAXTER is a trademark of Baxter International Inc.
For product information 1-800-933-0303
07-07-42-311 2003/12
Carton Label

Lot
Exp
10 x 200 mL Single Dose INTRAVIA Containers
200 mL Code 2J1445
Fluconazole Injection NDC 0338-6045-37
400 mg/200 mL
ISO-OSMOTIC SODIUM CHLORIDE DILUENT
Recommended storage: Store between 77°F (25°C) and 41°F (5°C). Brief exposure up to 104°F (40°C) does not adversely affect the product. Protect from freezing. Avoid excessive heat.
(01) 50303386045371
07-06-38-039
Baxter
| FLUCONAZOLE
fluconazole injection, solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA076766 | 08/14/2009 | |
| FLUCONAZOLE
fluconazole injection, solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA076766 | 08/14/2009 | |
| Labeler - Baxter Healthcare Corporation (005083209) |
| Registrant - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| BAXTER HEALTHCARE SA, dba Baxter Healthcare of Puerto Rico | 189326168 | MANUFACTURE | |