COSYNTROPIN
-
cosyntropin injection, solution
Sandoz Inc
----------
Cosyntropin Injection0.25 mg/mL
FOR DIAGNOSTIC USE ONLY
For Intravenous Use Only
DESCRIPTION
Cosyntropin Injection is a 1 mL sterile solution in vials containing 0.25 mg of cosyntropin, 0.82 mg sodium acetate trihydrate, 6.4 mg sodium chloride, 10 mg mannitol, 1 mg glacial acetic acid, and water for injection, USP. Administration is by intravenous injection. Cosyntropin is α 1-24 corticotropin, a synthetic subunit of ACTH. It is an open chain polypeptide containing, from the N terminus, the first 24 of the 39 amino acids of natural ACTH. The sequence of amino acids in the 1-24 compound is as follows:

CLINICAL PHARMACOLOGY
Cosyntropin injection exhibits the full corticosteroidogenic activity of natural ACTH. Various studies have shown that the biologic activity of ACTH resides in the N-terminal portion of the molecule and that the 1-20 amino acid residue is the minimal sequence retaining full activity. Partial or complete loss of activity is noted with progressive shortening of the chain beyond 20 amino acid residues. For example, the decrement from 20 to 19 results in a 70% loss of potency.
The pharmacologic profile of cosyntropin injection is similar to that of purified natural ACTH. It has been established that 0.25 mg of cosyntropin injection will stimulate the adrenal cortex maximally and to the same extent as 25 units of natural ACTH. This dose of cosyntropin injection will produce maximal secretion of 17-OH corticosteroids, 17-ketosteroids and/or 17-ketogenic steroids.
The extra-adrenal effects which natural ACTH and cosyntropin injection have in common include increased melanotropic activity, increased growth hormone secretion and an adipokinetic effect. These are considered to be without physiological or clinical significance.
Animal, human and synthetic ACTH (1-39) which all contain 39 amino acids exhibit similar immunologic activity. This activity resides in the C-terminal portion of the molecule and the 22-39 amino acid residues exhibit the greatest degree of antigenicity. In contrast, synthetic poly-peptides containing 1-19 or fewer amino acids have no detectable immunologic activity. Those containing 1-26, 1-24 or 1-23 amino acids have very little immunologic although full biologic activity. This property of cosyntropin injection assumes added importance in view of the known antigenicity of natural ACTH.
INDICATIONS AND USAGE
Cosyntropin injection is intended for use as a diagnostic agent in the screening of patients presumed to have adrenocortical insufficiency. Because of its rapid effect on the adrenal cortex it may be utilized to perform a 30-minute test of adrenal function (plasma cortisol response) as an office or outpatient procedure, using only 2 venipunctures (see DOSAGE AND ADMINISTRATION section).
Severe hypofunction of the pituitary-adrenal axis is usually associated with subnormal plasma cortisol values but a low basal level is not per se evidence of adrenal insufficiency and does not suffice to make the diagnosis. Many patients with proven insufficiency will have normal basal levels and will develop signs of insufficiency only when stressed. For this reason a criterion which should be used in establishing the diagnosis is the failure to respond to adequate corticotropin stimulation. When presumptive adrenal insufficiency is diagnosed by a subnormal cosyntropin injection test, further studies are indicated to determine if it is primary or secondary.
Primary adrenal insufficiency (Addison’s disease) is the result of an intrinsic disease process, such as tuberculosis within the gland. The production of adrenocortical hormones is deficient despite high ACTH levels (feedback mechanism). Secondary or relative insufficiency arises as the result of defective production of ACTH leading in turn to disuse atrophy of the adrenal cortex. It is commonly seen, for example, as result of corticosteroid therapy, Sheehan’s syndrome and pituitary tumors or ablation.
The differentiation of both types is based on the premise that a primarily defective gland cannot be stimulated by ACTH whereas a secondarily defective gland is potentially functional and will respond to adequate stimulation with ACTH. Patients selected for further study as the result of a subnormal cosyntropin injection test should be given a 3 or 4 day course of treatment with Repository Corticotropin Injection USP and then retested. Suggested doses are 40 USP units twice daily for 4 days or 60 USP units twice daily for 3 days. Under these conditions little or no increase in plasma cortisol levels will be seen in Addison’s disease whereas higher or even normal levels will be seen in cases with secondary adrenal insufficiency.
CONTRAINDICATION
The only contraindication to cosyntropin injection is a history of a previous adverse reaction to it.
PRECAUTIONS
General
Cosyntropin injection exhibits slight immunologic activity, does not contain animal protein and is therefore less risky to use than natural ACTH. Patients known to be sensitized to natural ACTH with markedly positive skin tests will, with few exceptions, react negatively when tested intradermally with cosyntropin injection. Most patients with a history of a previous hypersensitivity reaction to natural ACTH or a pre-existing allergic disease will tolerate cosyntropin injection. Despite this however, cosyntropin injection is not completely devoid of immunologic activity and hypersensitivity reactions including rare anaphylaxis are possible. Therefore, the physician should be prepared, prior to injection, to treat any possible acute hypersensitivity reaction.
Drug Interactions
Corticotropin may accentuate the electrolyte loss associated with diuretic therapy.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed to evaluate carcinogenic or mutagenic potential or impairment of fertility. A study in rats noted inhibition of reproductive function like natural ACTH.
Pregnancy
Pregnancy Category C
Safety in pregnant women has not been established. There are no adequate and well controlled studies of cosyntropin in pregnant women. Cosyntropin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether cosyntropin is excreted into milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from cosyntropin, caution should be exercised when cosyntropin injection is administered to a nursing woman.
Pediatric Use
(See DOSAGE AND ADMINISTRATION section.)
ADVERSE REACTIONS
Since cosyntropin injection is intended for diagnostic and not therapeutic use, adverse reactions other than a rare hypersensitivity reaction are not anticipated. A rare hypersensitivity reaction usually associated with a pre-existing allergic disease and/or a previous reaction to natural ACTH is possible. There have been rare reports of anaphylactic reaction. The following adverse reactions have been reported in patients after the administration of cosyntropin injection and the association has been neither confirmed nor refuted:
- bradycardia
- tachycardia
- hypertension
- peripheral edema
- rash
DOSAGE AND ADMINISTRATION
This product should not be given intramuscularly.
Cosyntropin injection may be administered as a direct intravenous injection when used as a rapid screening test of adrenal function. It may also be given as an intravenous infusion over a 4 to 8 hour period to provide a greater stimulus to the adrenal glands. Doses of cosyntropin injection 0.25 to 0.75 mg have been used in clinical studies and a maximal response noted with the smallest dose.
A suggested method for a rapid screening test of adrenal function has been described by Wood and Associates1. A control blood sample of 6 to 7 mL is collected in a heparinized tube. The drug product should be inspected visually for particulate matter and discoloration prior to injection. Inject 1 mL of cosyntropin injection. Discard any unused portion. In the pediatric population, aged 2 years or less, a dose of 0.125 mg will often suffice. A second blood sample is collected exactly 30 minutes later. Both blood samples should be refrigerated until sent to the laboratory for determination of the plasma cortisol response by some appropriate method. If it is not possible to send them to the laboratory or perform the fluorimetric procedure within 12 hours, then the plasma should be separated and refrigerated or frozen according to need.
Two alternative methods of administration are intravenous injection and infusion. Cosyntropin injection can be injected intravenously in 2 to 5 mL of 0.9 % Sodium Chloride Injection, USP over a 2-minute period. When given as an intravenous infusion: cosyntropin injection, 0.25 mg/mL may be added to either 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP and given at the rate of approximately 40 micrograms per hour over a 6-hour period. It should not be added to blood or plasma as it is apt to be inactivated by enzymes. Adrenal response may be measured in the usual manner by determining urinary steroid excretion before and after treatment or by measuring plasma cortisol levels before and at the end of the infusion. The latter is preferable because the urinary steroid excretion does not always accurately reflect the adrenal or plasma cortisol response to ACTH.
The usual normal response in most cases is an approximate doubling of the basal level, provided that the basal level does not exceed the normal range. Patients receiving cortisone, hydrocortisone or spironolactone should omit their pre-test doses on the day selected for testing. Patients taking inadvertent doses of cortisone or hydrocortisone on the test day and patients taking spironolactone or women taking drugs which contain estrogen may exhibit abnormally high basal plasma cortisol levels.
A paradoxical response may be noted in the cortisone or hydrocortisone group as seen in a decrease in plasma cortisol values following a stimulating dose of cosyntropin injection.
In the spironolactone or estrogen group only a normal incremental response is to be expected. Many patients with normal adrenal function, however, do not respond to the expected degree so that the following criteria have been established to denote a normal response:
- The control plasma cortisol level should exceed 5 micrograms/100 mL.
- The 30-minute level should show an increment of at least 7 micrograms/100 mL above the basal level.
- The 30-minute level should exceed 18 micrograms/100 mL. Comparable figures have been reported by Greig and co-workers2.
If the increase in plasma cortisol levels at 30 minutes is equivocal, a consideration should be given to repeat cortisol sampling at later time points (e.g. 60 and/or 90 minutes). In a study in normal volunteers, plasma cortisol levels peaked about 2.5 ± 0.5 hours after an intravenous injection of cosyntropin (see Fig. 1).
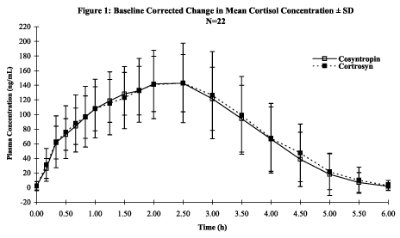
In patients with a raised plasma bilirubin or in patients where the plasma contains free hemoglobin, falsely high fluorescence measurements will result. The test may be performed at any time during the day but because of the physiological diurnal variation of plasma cortisol the criteria listed by Wood cannot apply. It has been shown that basal plasma cortisol levels and the post cosyntropin injection increment exhibit diurnal changes. However, the 30-minute plasma cortisol level remains unchanged throughout the day so that only this single criterion should be used3.
Parenteral drug products should be inspected visually for particulate matter and discoloration whenever solution and container permit. Unused cosyntropin injection should be discarded.
HOW SUPPLIED
NDC 0781-3052-95 Cosyntropin Injection 0.25 mg/mL, Box of 10 - 1 mL vials
Store refrigerated between 2°-8°C (36°-46°F). Protect from light. Protect from freezing.
Cosyntropin injection is intended as a single dose injection and contains no antimicrobial preservative. Any unused portion should be discarded.
REFERENCES
- Wood, J.B. et al. LANCET 1.243, 1965.
- Greig, W.R. et al. J. ENDOCR 34.411, 1966.
- McGill, P.E. et al. ANN RHEUM DIS 26.123, 1967.
07-2009M
1006847
Manufactured in Canada by
Sandoz Canada Inc. for
Sandoz Inc.
Princeton, NJ 08540
PRINCIPAL DISPLAY PANEL

| COSYNTROPIN
cosyntropin injection, solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA022028 | 02/21/2008 | |
| Labeler - Sandoz Inc (110342024) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sandoz Canada Inc | 244062071 | MANUFACTURE | |