HEPARIN- heparin sodium injection, solution
Hospira, Inc.
----------
100 USP Units/mL
For Heparin-Saline Lock of
Indwelling Venipuncture Devices
Ansyr® Unit of Use Plastic Syringe
SOLUTION IS INTENDED FOR
MAINTENANCE OF PATENCY OF
INTRAVENOUS INJECTION DEVICES
ONLY AND IS NOT TO BE USED FOR
ANTICOAGULANT THERAPY.
DESCRIPTION
Heparin Lock Flush Solution, USP is a sterile nonpyrogenic preparation of Heparin Sodium Injection, USP with sufficient sodium chloride to make it isotonic in water for injection.
Each milliliter (mL) contains: Heparin sodium, 100 USP units (derived from porcine intestinal mucosa); edetate disodium, anhydrous 0.1 mg added as a stabilizer; and sodium chloride, 4.5 mg to render the solution isotonic in water for injection. May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. pH 6.1 (5.0 to 7.5).
The solution contains no bacteriostat, antimicrobial agent or added buffer. Each disposable syringe is intended only for one-time use.
Heparin Lock Flush Solution, USP is intended for maintenance of patency of intravenous injection devices only and is not to be used for anticoagulant therapy.
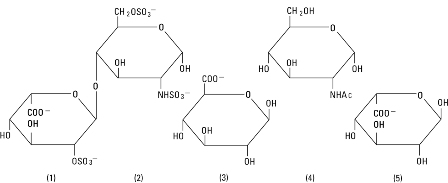
Heparin Sodium, USP is a heterogenous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans having anticoagulant properties. Although others may be present, the main sugars occurring in heparin are: (1) α-L-iduronic acid 2-sulfate, (2) 2-deoxy-2-sulfamino-α-D-glucose 6-sulfate, (3) β-D-glucuronic acid, (4) 2-acetamido-2-deoxy-α-D-glucose, and (5) α-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2)>(1)>(4)>(3)>(5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.
Structure of Heparin Sodium (representative subunits):
Sodium Chloride, USP is chemically designated NaCl, a white crystalline compound freely soluble in water.
The plastic syringe is molded from a specially formulated polypropylene. Water permeates from inside the container at an extremely slow rate which will have an insignificant effect on solution concentration over the expected shelf life. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the syringe material.
CLINICAL PHARMACOLOGY
Heparin inhibits reactions that lead to the clotting of blood and the formation of fibrin clots both in vitro and in vivo. Heparin acts at multiple sites in the normal coagulation system. Small amounts of heparin in combination with antithrombin III (heparin cofactor) can inhibit thrombosis by inactivating activated Factor X and inhibiting the conversion of prothrombin to thrombin. Once active thrombosis has developed, larger amounts of heparin can inhibit further coagulation by inactivating thrombin and preventing the conversion of fibrinogen to fibrin. Heparin also prevents the formation of a stable fibrin clot in inhibiting the activation of the fibrin stabilizing factor.
Bleeding time is usually unaffected by heparin. Clotting time is prolonged by full therapeutic doses of heparin; in most cases, it is not measurably affected by low doses of heparin.
Patients over 60 years of age, following similar doses of heparin, may have higher plasma levels of heparin and longer activated partial thromboplastin times (APTTs) compared with patients under 60 years of age.
Peak plasma levels of heparin are achieved 2 to 4 hours following subcutaneous administration, although there are considerable individual variations. Loglinear plots of heparin plasma concentrations with time for a wide range of dose levels are linear which suggests the absence of zero order processes. Liver and the reticuloendothelial system are the site of biotransformation. The biphasic elimination curve, a rapidly declining alpha phase (t½= 10′) and after the age of 40 a slower beta phase, indicates uptake in organs. The absence of a relationship between anticoagulant half-life and concentration of half-life may reflect factors such as protein binding of heparin.
Heparin does not have fibrinolytic activity; therefore, it will not lyse existing clots.
Heparin Lock Flush Solution does not induce systemic anticoagulant action at single doses of 100 USP units per mL when used for maintenance of patency of intravenous injection devices. It may interfere with laboratory tests on blood samples withdrawn from such devices, unless the volume of in situ heparin-saline, equal to that of the priming volume of the catheter, is aspirated and discarded before such samples are taken.
Isotonic concentrations of sodium chloride are suitable for parenteral replacement of chloride losses that exceed or equal the sodium loss. Hypotonic concentrations of sodium chloride are suited for parenteral maintenance of water requirements when only small quantities of salt are desired.
Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl‾) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Chloride (Cl‾) has an integral role in buffering action when oxygen and carbon dioxide exchange occurs in the red blood cells. The distribution and excretion of sodium (Na+) are largely under the control of the kidney which maintains a balance between intake and output.
INDICATIONS AND USAGE
Heparin Lock Flush Solution, USP is indicated only to maintain patency of an intravenous injection device. It may be used following initial placement of the device in the vein, after each injection of a medication or after withdrawal of blood for laboratory analysis.
The solution is not to be used for anticoagulation therapy.
To prevent clot formation in a central venous catheter or peripherally-inserted central venous catheter following its proper insertion, Heparin Lock Flush Solution, 100 Units/mL is injected into the catheter (or into each unused lumen of a multiple-lumen catheter) in a quantity sufficient to fill the entire catheter or lumen of a multiple-lumen catheter to the tip (see catheter manufacturer’s labeling for specific catheter lumen volumes). This solution should be replaced each time the catheter (or catheter lumen in a multiple lumen catheter) is used. Aspirate before administering any solution in order to confirm catheter patency. If the drug to be administered is incompatible with heparin, the entire catheter or catheter lumen should be flushed with sterile water or normal saline before and after the medication is administered. Following the second flush, Heparin Lock Flush Solution may be reinstilled into the set. The catheter manufacturer’s instructions should be consulted for specifics concerning the catheter in use at a given time.
CONTRAINDICATIONS
Heparin sodium should not be used in patients:
With hypersensitivity to heparin;
With an uncontrollable active bleeding state (see WARNINGS), except when this is due to disseminated intravascular coagulation;
With inability to perform suitable blood-coagulation tests, e.g., whole-blood clotting time, partial thromboplastin time, etc., at required intervals. There is usually no need to monitor effect of low-dose heparin in patients with normal coagulation parameters.
WARNINGS
Heparin Lock Flush Solution, USP is not intended for intramuscular use, systemic anticoagulation or injection by any parenteral route of administration. Repeated flushing of a catheter device with the Heparin 100 USP Units/mL may result in a systemic anticoagulant effect.
Hypersensitivity: Patients with documented hypersensitivity to heparin should be given the drug only in clearly life-threatening situations.
Hemorrhage: Hemorrhage can occur at virtually any site in patients receiving heparin. An unexplained fall in hematocrit, fall in blood pressure, or any other unexplained symptom should lead to serious consideration of a hemorrhagic event.
Heparin sodium should be used with extreme caution in disease states in which there is increased danger of hemorrhage. Some of the conditions in which increased danger of hemorrhage exists are:
Cardiovascular — Subacute bacterial endocarditis. Severe hypertension.
Surgical — During and immediately following (a) spinal tap or spinal anesthesia or (b) major surgery, especially involving the brain, spinal cord or eye.
Hematologic — Conditions associated with increased bleeding tendencies, such as hemophilia, thrombocytopenia, and some vascular purpuras.
Gastrointestinal — Ulcerative lesions and continuous tube drainage of the stomach or small intestine.
Other — Menstruation, liver disease with impaired hemostasis.
Coagulation Testing: If the coagulation test is unduly prolonged or if hemorrhage occurs, heparin sodium should be discontinued promptly. Heparin solutions having a concentration of 100 USP Heparin Units/mL will alter the results of blood coagulation tests.
Thrombocytopenia: Thrombocytopenia has been reported to occur in patients receiving heparin with a reported incidence of 0 to 30%. Mild thrombocytopenia (count greater than 100,000/mm3) may remain stable or reverse even if heparin is continued. However, thrombocytopenia of any degree should be monitored closely. If the count falls below 100,000/mm3 or if recurrent thrombosis develops (see White Clot Syndrome, PRECAUTIONS), the heparin product should be discontinued. If continued heparin therapy is essential, administration of heparin from a different organ source can be reinstituted with caution.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
PRECAUTIONS
Do not use unless solution is clear and container is undamaged. Not for multiple dosing. After single use, discard unused portion in the Ansyr® unit of use 5 mL plastic syringe (100 USP Units/mL).
Since heparin sodium in this product is derived from animal tissue, it should be used with caution in patients with a history of allergy.
Interference with Laboratory Tests:
Heparin interferes with competitive protein binding methods for serum thyroxine determinations resulting in falsely elevated levels.
Heparin Lock Flush Solution may interfere with laboratory analyses or alter the results of blood chemistry tests such as glucose, serum sodium and serum chloride, blood coagulation studies, etc. (see CLINICAL PHARMACOLOGY).
The following information which pertains to the use of heparin sodium as a systemic anticoagulant is included as a matter of interest only since it is not known to apply to the use of the drug for heparin lock.
General:
a. White Clot Syndrome:
It has been reported that patients on heparin may develop new thrombus formation in association with thrombocytopenia resulting from irreversible aggregation of platelets induced by heparin, the so-called “white clot syndrome”. The process may lead to severe thromboembolic complications like skin necrosis, gangrene of the extremities that may lead to amputation, myocardial infarction, pulmonary embolism, stroke, and possibly death. Therefore, heparin administration should be promptly discontinued if a patient develops new thrombosis in association with thrombocytopenia.
b. Heparin Resistance:
Increased resistance to heparin is frequently encountered in fever, thrombosis, thrombophlebitis, infections with thrombosing tendencies, myocardial infarction, cancer and in postsurgical patients.
c. Increased Risk in Older Patients, Especially Women:
A higher incidence of bleeding has been reported in patients, particularly women, over 60 years of age.
Drug Interactions:
Oral anticoagulants: Heparin sodium may prolong the one-stage prothrombin time. Therefore, when heparin sodium is given with dicumarol or warfarin sodium, a period of at least 5 hours after the last intravenous dose should elapse before blood is drawn if a valid PROTHROMBIN time is to be obtained.
Platelet inhibitors: Drugs such as acetylsalicylic acid, dextran, phenylbutazone, ibuprofen, indomethacin, dipyridamole, hydroxychloroquine and others that interfere with platelet-aggregation reactions (the main hemostatic defense of heparinized patients) may induce bleeding and should be used with caution in patients receiving heparin sodium.
Other interactions: Digitalis, tetracyclines, nicotine, anti-histamines or I.V. nitroglycerin may partially counteract the anticoagulant action of heparin sodium.
Drug/Laboratory Interactions:
Hyperaminotransferasemia: Significant elevations of aminotransferase (SGOT [S-AST] and SGPT [S-ALT]) levels have occurred in a high percentage of patients (and healthy subjects) who have received heparin. Since aminotransferase determinations are important in the differential diagnosis of myocardial infarction, liver disease, and pulmonary emboli, rises that might be caused by drugs (like heparin) should be interpreted with caution.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No long-term studies in animals have been performed to evaluate carcinogenic potential of heparin. Also, no reproduction studies in animals have been performed concerning mutagenesis or impairment of fertility.
Pregnancy:
Teratogenic Effects: Pregnancy Category C. Animal reproduction studies have not been conducted with heparin sodium or sodium chloride. It is also not known whether heparin sodium or sodium chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Heparin sodium or sodium chloride should be given to a pregnant woman only if clearly needed.
Nonteratogenic Effects: Heparin does not cross the placental barrier.
Nursing Mothers: Heparin is not excreted in human milk.
Pediatric Use: Safety and effectiveness of the 100 USP Units/mL Heparin Lock Flush Solution, USP, in pediatric patients have not been established.
Geriatric Use: A higher incidence of bleeding has been reported in patients over 60 years of age, especially women (see PRECAUTIONS, General). Clinical studies indicate that lower doses of heparin may be indicated in these patients (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
Heparin Lock Flush Solution is not known to cause adverse local or systemic effects of any kind. Although a remote possibility of hypersensitivity reaction exists with entry of extremely small subtherapeutic amounts of the solution into the circulation, such an occurrence has not been reported.
DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Slight discoloration does not alter potency (see PRECAUTIONS).
Heparin Lock Flush Solution, USP 100 USP Units/mL, is injected as a single dose into an intravenous injection device using a volume of solution equivalent to that of the indwelling venipuncture device (see catheter manufacturer’s labeling for specific catheter lumen volumes).
A single dose should be injected following venipuncture when the indwelling device is not to be used immediately. After each use of the indwelling venipuncture device for injection or infusion of medication, or withdrawal of blood samples, another dose (using a volume of solution equivalent to that of the indwelling venipuncture device) should be injected to restore the effectiveness of the heparin lock. The amount of heparin solution in each single dose is sufficient to prevent clotting within the lumen of indwelling venipuncture devices for up to twenty-four hours.
When the indwelling device is used to administer a drug which is incompatible with heparin, the entire heparin lock set should be flushed with 0.9% Sodium Chloride Injection, USP before and after the medication is administered. Following the second flush, another dose of heparin solution should be injected to restore the effectiveness of the heparin lock.
When the indwelling device is used for repeated withdrawal of blood samples for laboratory analyses and the presence of heparin or saline is likely to interfere with or alter results of the desired blood tests, the in situ heparin flush solution should be cleared from the device by aspirating and discarding a volume of solution equivalent to that of the indwelling venipuncture device before the desired blood sample is drawn. (See PRECAUTIONS.)
HOW SUPPLIED
Heparin Lock Flush Solution is available as follows:
|
List |
Container |
Concentration |
Fill |
Quantity |
|
3454 |
Ansyr® Syringe with LifeShield® male luer lock adapter |
100 USP Units/mL |
5 mL |
Box of 1 |
|
3454 |
Ansyr® Syringe with LifeShield® male luer lock adapter |
100 USP Units/mL |
5 mL |
Box of 50 |
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
May be used with sharp needles. May also be used with the LifeShield® Blunt Cannula.
INSTRUCTIONS FOR USE
For access to a standard luer injection site: Remove luer cover from syringe. Hold plunger and push barrel forward to relieve any resistance that may be present. Pull the barrel down until air is expelled from the syringe. Attach syringe to luer access device. Inject medication. (See DOSAGE AND ADMINISTRATION section.) Discard unused portion after initial use. Discard syringe per health care provider policy.
For injection using a sharp needle or blunt cannula: Remove luer cover from syringe. Attach desired compatible sharp needle or blunt cannula to luer. Hold plunger and push barrel forward to relieve any resistance that may be present. Pull the barrel down until air is expelled from the syringe. Inject medication. (See DOSAGE AND ADMINISTRATION section.) Discard unused portion after initial use. Discard syringe per health care provider policy.
Revised: October, 2004
©Hospira 2004 EN-0497 Printed in USA
HOSPIRA, INC., LAKE FOREST, IL 60045 USA
| HEPARIN
heparin sodium injection, solution |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |