COLD AND MUCUS RELIEF COUGH EXPECTORANT- antimony pentasulfide, potassium iodide and polygala senega root syrup
Similasan AG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Uses:
Temporarily relieves symptoms associated with the common cold, such as: cough, fever, chest and head congestion.
Warnings:
- Consult a physician if symptoms persist for mroe than 7 days or worsen.
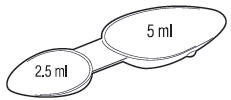
- Do not exceed recommended dosage.
| COLD AND MUCUS RELIEF COUGH EXPECTORANT
antimonium sulphuratum aureum and kali iodatum and senega officinalis syrup |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Similasan AG (481545754) |
Revised: 07/2013
Document Id: a29e415a-64ee-4789-8659-60590493c70f
Set id: 0d9fe55f-7ff1-49ba-b9b0-c5ee461c65a1
Version: 5
Effective Time: 20130708
Similasan AG