JENTADUETO- linagliptin and metformin hydrochloride tablet, film coated
Boehringer Ingelheim Pharmaceuticals, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use JENTADUETO safely and effectively. See full prescribing information for JENTADUETO.
Jentadueto® (linagliptin and metformin hydrochloride) tablets Initial U.S. Approval: 2012 WARNING: RISK OF LACTIC ACIDOSISSee full prescribing information for complete boxed warning.
RECENT MAJOR CHANGESINDICATIONS AND USAGEJENTADUETO is a dipeptidyl peptidase-4 (DPP-4) inhibitor and biguanide combination product indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both linagliptin and metformin is appropriate (1.1) Important limitations of use: DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSTablets: CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257 or 1-800-459-9906 TTY, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 9/2013 |
FULL PRESCRIBING INFORMATION
WARNING: RISK OF LACTIC ACIDOSIS
Lactic acidosis is a rare, but serious, complication that can occur due to metformin accumulation. The risk increases with conditions such as renal impairment, sepsis, dehydration, excessive alcohol intake, hepatic impairment, and acute congestive heart failure.
The onset is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress.
Laboratory abnormalities include low pH, increased anion gap, and elevated blood lactate.
If acidosis is suspected, JENTADUETO should be discontinued and the patient hospitalized immediately [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
1.1 Indication
JENTADUETO tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both linagliptin and metformin is appropriate [see Dosage and Administration (2.1) and Clinical Studies (14.1)].
1.2 Important Limitations of Use
JENTADUETO should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis, as it would not be effective in these settings.
JENTADUETO has not been studied in patients with a history of pancreatitis. It is unknown whether patients with a history of pancreatitis are at an increased risk for the development of pancreatitis while using JENTADUETO [see Warnings and Precautions (5.2)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
The dosage of JENTADUETO should be individualized on the basis of both effectiveness and tolerability, while not exceeding the maximum recommended dose of 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily. JENTADUETO should be given twice daily with meals. Dose escalation should be gradual to reduce the gastrointestinal (GI) side effects associated with metformin use. For available dosage forms and strengths see [Dosage Forms and Strengths (3)].
- In patients currently not treated with metformin, initiate treatment with 2.5 mg linagliptin/500 mg metformin hydrochloride twice daily
- In patients already treated with metformin, start with 2.5 mg linagliptin and the current dose of metformin taken at each of the two daily meals (e.g., a patient on metformin 1000 mg twice daily would be started on 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily with meals).
- Patients already treated with linagliptin and metformin individual components may be switched to JENTADUETO containing the same doses of each component.
No studies have been performed specifically examining the safety and efficacy of JENTADUETO in patients previously treated with other oral antihyperglycemic agents and switched to JENTADUETO. Any change in therapy of type 2 diabetes mellitus should be undertaken with care and appropriate monitoring as changes in glycemic control can occur.
2.2 Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
When JENTADUETO is used in combination with an insulin secretagogue (e.g., sulfonylurea) or with insulin, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia [see Warnings and Precautions (5.5)].
3 DOSAGE FORMS AND STRENGTHS
JENTADUETO is a combination of linagliptin and metformin hydrochloride. JENTADUETO tablets are available in the following dosage forms and strengths:
- 2.5 mg linagliptin/500 mg metformin hydrochloride tablets are light yellow, oval, biconvex tablets debossed with “D2/500” on one side and the Boehringer Ingelheim logo on the other side
- 2.5 mg linagliptin/850 mg metformin hydrochloride tablets are light orange, oval, biconvex tablets debossed with “D2/850” on one side and the Boehringer Ingelheim logo on the other side
- 2.5 mg linagliptin/1000 mg metformin hydrochloride tablets are light pink, oval, biconvex tablets debossed with “D2/1000” on one side and the Boehringer Ingelheim logo on the other side
4 CONTRAINDICATIONS
JENTADUETO is contraindicated in patients with:
- Renal impairment (e.g., serum creatinine ≥1.5 mg/dL for men, ≥1.4 mg/dL for women, or abnormal creatinine clearance) which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia [see Warnings and Precautions (5.1, 5.3)]
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis. Diabetic ketoacidosis should be treated with insulin [see Warnings and Precautions (5.1)]
- A history of hypersensitivity reaction to linagliptin (such as urticaria, angioedema, or bronchial hyperreactivity) or metformin [see Adverse Reactions (6.1)]
5 WARNINGS AND PRECAUTIONS
5.1 Lactic Acidosis
Lactic acidosis is a serious, metabolic complication that can occur due to metformin accumulation during treatment with JENTADUETO and is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels of >5 µg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin is approximately 0.03 cases/1000 patient-years, (with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal impairment, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, particularly when accompanied by hypoperfusion and hypoxemia due to unstable or acute failure, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal impairment and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in any patient unless measurement of creatinine clearance demonstrates that renal function is not reduced. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should be avoided in patients with clinical or laboratory evidence of hepatic impairment. Patients should be cautioned against excessive alcohol intake when taking metformin, since alcohol potentiates the effects of metformin on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure necessitating restricted intake of food or fluids. Use of topiramate, a carbonic anhydrase inhibitor, in epilepsy and migraine prophylaxis may cause dose-dependent metabolic acidosis and may exacerbate the risk of metformin-induced lactic acidosis [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
The onset of lactic acidosis is often subtle, and accompanied by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. More severe acidosis may be associated with signs such as hypothermia, hypotension, and resistant bradyarrhythmias. Patients should be educated to recognize and promptly report these symptoms. If present, JENTADUETO should be discontinued until lactic acidosis is ruled out. Gastrointestinal symptoms, which are commonly reported during initiation of metformin therapy are less frequently observed in subjects on a chronic, stable, dose of metformin. Gastrointestinal symptoms in subjects on chronic, stable, dose of metformin could be caused by lactic acidosis or other serious disease.
To rule out lactic acidosis, serum electrolytes, ketones, blood glucose, blood pH, lactate levels, and blood metformin levels may be useful. Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be due to other mechanisms, such as poorly-controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling.
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia). Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and supportive measures promptly instituted. Metformin is dialyzable (clearance of up to 170 mL/min under good hemodynamic conditions) and prompt hemodialysis is recommended to remove the accumulated metformin and correct the metabolic acidosis. Such management often results in prompt reversal of symptoms and recovery [see Boxed Warning].
5.2 Pancreatitis
There have been postmarketing reports of acute pancreatitis, including fatal pancreatitis, in patients taking linagliptin. Take careful notice of potential signs and symptoms of pancreatitis. If pancreatitis is suspected, promptly discontinue JENTADUETO and initiate appropriate management. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using JENTADUETO.
5.3 Monitoring of Renal Function
Although linagliptin undergoes minimal renal excretion, metformin is known to be substantially excreted by the kidney. The risk of metformin accumulation and lactic acidosis increases with the degree of renal impairment. Therefore, JENTADUETO is contraindicated in patients with renal impairment.
Before initiation of therapy with JENTADUETO and at least annually thereafter, renal function should be assessed and verified to be normal. In patients in whom development of renal impairment is anticipated (e.g., elderly), renal function should be assessed more frequently and JENTADUETO discontinued if evidence of renal impairment is present.
Linagliptin may be continued as a single entity tablet at the same total daily dose of 5 mg if JENTADUETO is discontinued due to evidence of renal impairment. No dose adjustment of linagliptin is recommended in patients with renal impairment.
Use of concomitant medications that may affect renal function or metformin disposition:
Concomitant medication(s) that may affect renal function or result in significant hemodynamic change or interfere with the disposition of metformin should be used with caution [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Radiological studies and surgical procedures:
Radiologic studies involving the use of intravascular iodinated contrast materials (e.g., intravenous urogram, intravenous cholangiography, angiography, and computed tomography) can lead to acute alteration of renal function and have been associated with lactic acidosis in patients receiving metformin. Therefore, in patients in whom any such study is planned, JENTADUETO should be temporarily discontinued at the time of or prior to the procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been confirmed to be normal.
JENTADUETO should be temporarily discontinued for any surgical procedure (except minor procedures not associated with restricted intake of food and fluids) and should not be restarted until the patient's oral intake has resumed and renal function has been evaluated as normal.
5.4 Impaired Hepatic Function
Because impaired hepatic function has been associated with some cases of lactic acidosis with metformin therapy, JENTADUETO should generally be avoided in patients with clinical or laboratory evidence of hepatic disease [see Warnings and Precautions (5.1)].
5.5 Use with Medications Known to Cause Hypoglycemia
Insulin secretagogues and insulin are known to cause hypoglycemia. The use of linagliptin in combination with an insulin secretagogue (e.g., sulfonylurea) was associated with a higher rate of hypoglycemia compared with placebo in a clinical trial [see Adverse Reactions (6.1)]. The use of linagliptin in combination with insulin in subjects with severe renal impairment was associated with a higher rate of hypoglycemia [see Adverse Reactions (6.1)]. Therefore, a lower dose of the insulin secretagogue or insulin may be required to reduce the risk of hypoglycemia when used in combination with JENTADUETO [see Dosage and Administration (2.2)].
Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, but could occur when caloric intake is deficient, when strenuous exercise is not compensated by caloric supplementation, or during concomitant use with other glucose-lowering agents (such as SUs and insulin) or ethanol. Elderly, debilitated, or malnourished patients, and those with adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be difficult to recognize in the elderly, and in people who are taking β-adrenergic blocking drugs.
5.6 Vitamin B12 Levels
In controlled, 29-week clinical trials of metformin, a decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of metformin-treated patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia or neurologic manifestations due to the short duration (<1 year) of the clinical trials. This risk may be more relevant to patients receiving long-term treatment with metformin, and adverse hematologic and neurologic reactions have been reported postmarketing. The decrease in vitamin B12 levels appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Measurement of hematologic parameters on an annual basis is advised in patients on JENTADUETO and any apparent abnormalities should be appropriately investigated and managed. Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels. In these patients, routine serum vitamin B12 measurement at 2- to 3-year intervals may be useful.
5.7 Alcohol Intake
Alcohol is known to potentiate the effect of metformin on lactate metabolism. Patients, therefore, should be warned against excessive alcohol intake while receiving JENTADUETO [see Warnings and Precautions (5.1)].
5.8 Hypoxic States
Cardiovascular collapse (shock) from whatever cause (e.g., acute congestive heart failure, acute myocardial infarction, and other conditions characterized by hypoxemia) have been associated with lactic acidosis and may also cause prerenal azotemia. When such events occur in patients on JENTADUETO therapy, the drug should be promptly discontinued [see Warnings and Precautions (5.1)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of concomitantly administered linagliptin (daily dose 5 mg) and metformin (mean daily dose of approximately 1800 mg) has been evaluated in 2816 patients with type 2 diabetes mellitus treated for ≥12 weeks in clinical trials.
Three placebo-controlled studies with linagliptin + metformin were conducted: 2 studies were 24 weeks in duration, 1 study was 12 weeks in duration. In the 3 placebo-controlled clinical studies, adverse events which occurred in ≥5% of patients receiving linagliptin + metformin (n=875) and were more common than in patients given placebo + metformin (n=539) included nasopharyngitis (5.7% vs 4.3%).
In a 24-week factorial design study, adverse events reported in ≥5% of patients receiving linagliptin + metformin and were more common than in patients given placebo are shown in Table 1.
| Placebo n=72 | Linagliptin Monotherapy n=142 | Metformin Monotherapy n=291 | Combination of Linagliptin with Metformin n=286 |
|
| n (%) | n (%) | n (%) | n (%) | |
| Nasopharyngitis | 1 (1.4) | 8 (5.6) | 8 (2.7) | 18 (6.3) |
| Diarrhea | 2 (2.8) | 5 (3.5) | 11 (3.8) | 18 (6.3) |
Other adverse reactions reported in clinical studies with treatment of linagliptin + metformin were hypersensitivity (e.g., urticaria, angioedema, or bronchial hyperreactivity), cough, decreased appetite, nausea, vomiting, pruritus, and pancreatitis.
Adverse reactions reported in ≥2% of patients treated with linagliptin 5 mg and more commonly than in patients treated with placebo included: nasopharyngitis (7.0% vs 6.1%), diarrhea (3.3% vs 3.0%), and cough (2.1% vs 1.4%).
Rates for other adverse reactions for linagliptin 5 mg vs placebo when linagliptin was used in combination with specific anti-diabetic agents were: urinary tract infection (3.1% vs 0%) and hypertriglyceridemia (2.4% vs 0%) when linagliptin was used as add-on to sulfonylurea; hyperlipidemia (2.7% vs 0.8%) and weight increased (2.3% vs 0.8%) when linagliptin was used as add-on to pioglitazone; and constipation (2.1% vs 1%) when linagliptin was used as add-on to basal insulin therapy.
Other adverse reactions reported in clinical studies with treatment of linagliptin monotherapy were hypersensitivity (e.g., urticaria, angioedema, localized skin exfoliation, or bronchial hyperreactivity) and myalgia. In the clinical trial program, pancreatitis was reported in 15.2 cases per 10,000 patient year exposure while being treated with linagliptin compared with 3.7 cases per 10,000 patient year exposure while being treated with comparator (placebo and active comparator, sulfonylurea). Three additional cases of pancreatitis were reported following the last administered dose of linagliptin.
The most common adverse reactions due to initiation of metformin are diarrhea, nausea/vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache.
Long-term treatment with metformin has been associated with a decrease in vitamin B12 absorption which may very rarely result in clinically significant vitamin B12 deficiency (e.g., megaloblastic anemia) [see Warnings and Precautions (5.5)].
Hypoglycemia
Linagliptin/Metformin
In a 24-week factorial design study, hypoglycemia was reported in 4 (1.4%) of 286 subjects treated with linagliptin + metformin, 6 (2.1%) of 291 subjects treated with metformin, and 1 (1.4%) of 72 subjects treated with placebo. When linagliptin was administered in combination with metformin and a sulfonylurea, 181 (22.9%) of 792 patients reported hypoglycemia compared with 39 (14.8%) of 263 patients administered placebo in combination with metformin and sulfonylurea. Adverse reactions of hypoglycemia were based on all reports of hypoglycemia. A concurrent glucose measurement was not required or was normal in some patients. Therefore, it is not possible to conclusively determine that all these reports reflect true hypoglycemia.
In the study of patients receiving linagliptin as add-on therapy to a stable dose of insulin for up to 52 weeks (n=1261), no significant difference in the incidence of investigator reported hypoglycemia, defined as all symptomatic or asymptomatic episodes with a self measured blood glucose ≤70 mg/dL, was noted between the linagliptin- (31.4%) and placebo- (32.9%) treated groups.
Linagliptin was compared to placebo as add-on to pre-existing antidiabetic therapy over 52 weeks in 133 patients with severe renal impairment (estimated GFR <30 mL/min). For the initial 12 weeks of the study, background antidiabetic therapy was kept stable and included insulin, sulfonylurea, glinides, and pioglitazone. For the remainder of the trial, dose adjustments in antidiabetic background therapy were allowed.
In general, the incidence of adverse events including severe hypoglycemia was similar to those reported in other linagliptin trials. The observed incidence of hypoglycemia was higher (linagliptin, 63% compared to placebo, 49%) due to an increase in asymptomatic hypoglycemic events especially during the first 12 weeks when background glycemic therapies were kept stable. Ten linagliptin-treated patients (15%) and 11 placebo-treated patients (17%) reported at least one episode of confirmed symptomatic hypoglycemia (accompanying finger stick glucose ≤54 mg/dL). During the same time period, severe hypoglycemic events, defined as an event requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions, were reported in 3 (4.4%) linagliptin-treated patients and 3 (4.6%) placebo-treated patients. Events that were considered life-threatening or required hospitalization were reported in 2 (2.9%) patients on linagliptin and 1 (1.5%) patient on placebo.
Renal function as measured by mean eGFR and creatinine clearance did not change over 52 weeks’ treatment compared to placebo.
Changes in laboratory findings were similar in patients treated with linagliptin + metformin compared to patients treated with placebo + metformin. Changes in laboratory values that occurred more frequently in the linagliptin + metformin group and ≥1% more than in the placebo group were not detected.
No clinically meaningful changes in vital signs were observed in patients treated with linagliptin.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during postapproval use of linagliptin. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
7.1 Drug Interactions with Metformin
Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, or vancomycin) that are eliminated by renal tubular secretion theoretically have the potential for interaction with metformin by competing for common renal tubular transport systems. Although such interactions remain theoretical (except for cimetidine), careful patient monitoring and dose adjustment of JENTADUETO and/or the interfering drug is recommended in patients who are taking cationic medications that are excreted via the proximal renal tubular secretory system [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
Topiramate or other carbonic anhydrase inhibitors (e.g., zonisamide, acetazolamide or dichlorphenamide) frequently decrease serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis. Concomitant use of these drugs may induce metabolic acidosis. Use these drugs with caution in patients treated with JENTADUETO, as the risk of lactic acidosis may increase [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 Drug Interactions with Linagliptin
Inducers of P-glycoprotein and CYP3A4 Enzymes
Rifampin decreased linagliptin exposure, suggesting that the efficacy of linagliptin may be reduced when administered in combination with a strong P-gp inducer or CYP 3A4 inducer. As JENTADUETO is a fixed-dose combination of linagliptin and metformin, use of alternative treatments (not containing linagliptin) is strongly recommended when concomitant treatment with a strong P-gp or CYP 3A4 inducer is necessary [see Clinical Pharmacology (12.3)].
7.3 Drugs Affecting Glycemic Control
Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are administered to a patient receiving JENTADUETO, the patient should be closely observed to maintain adequate glycemic control [see Clinical Pharmacology (12.3)]. When such drugs are withdrawn from a patient receiving JENTADUETO, the patient should be observed closely for hypoglycemia.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There are no adequate and well controlled studies in pregnant women with JENTADUETO or its individual components, and some clinical data is available for metformin which indicate that the risk for major malformations was not increased when metformin is taken during the first trimester in pregnancy. In addition, metformin was not associated with increased perinatal complications. Nevertheless, because these clinical data cannot rule out the possibility of harm, JENTADUETO should be used during pregnancy only if clearly needed.
JENTADUETO was not teratogenic when administered to Wistar Han rats during the period of organogenesis at doses similar to clinical exposure. At higher maternally toxic doses (9 and 23 times the clinical dose based on exposure), the metformin component of the combination was associated with an increased incidence of fetal rib and scapula malformations.
Linagliptin was not teratogenic when administered to pregnant Wistar Han rats and Himalayan rabbits during the period of organogenesis at doses up to 240 mg/kg and 150 mg/kg, respectively. These doses represent approximately 943 times the clinical dose in rats and 1943 times the clinical dose in rabbits, based on exposure. No functional, behavioral, or reproductive toxicity was observed in offspring of female Wistar Han rats when administered linagliptin from gestation day 6 to lactation day 21 at a dose 49 times the maximum recommended human dose, based on exposure.
Linagliptin crosses the placenta into the fetus following oral dosing in pregnant rats and rabbits.
Metformin has been studied for embryofetal effects in 2 rat strains and in rabbits. Metformin was not teratogenic in Sprague Dawley rats up to 600 mg/kg or in Wistar Han rats up to 200 mg/kg (2-3 times the clinical dose based on body surface area or exposure, respectively). At higher maternally toxic doses (9 and 23 times the clinical dose based on exposure), an increased incidence of rib and scapula skeletal malformations was observed in the Wistar Han strain. Metformin was not teratogenic in rabbits at doses up to 140 mg/kg (similar to clinical dose based on body surface area).
Metformin administered to female Sprague Dawley rats from gestation day 6 to lactation day 21 up to 600 mg/kg/day (2 times the maximum clinical dose based on body surface area) had no effect on prenatal or postnatal development of offspring.
Metformin crosses the placenta into the fetus in rats and humans.
8.3 Nursing Mothers
No studies in lactating animals have been conducted with the combined components of JENTADUETO. In studies performed with the individual components, both linagliptin and metformin were secreted in the milk of lactating rats. It is not known whether linagliptin is excreted in human milk. Metformin is excreted in human milk in low concentrations. Because the potential for hypoglycemia in nursing infants may exist, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of JENTADUETO in pediatric patients under 18 years of age have not been established.
8.5 Geriatric Use
Linagliptin is minimally excreted by the kidney; however, metformin is substantially excreted by the kidney. Considering that aging can be associated with reduced renal function, JENTADUETO should be used with caution as age increases [see Warnings and Precautions (5.1, 5.3) and Clinical Pharmacology (12.3)].
There were 4040 type 2 diabetes patients treated with linagliptin 5 mg from 15 clinical trials of linagliptin; 1085 (27%) patients were 65 years and over, while 131 (3%) were 75 years and over. Of these patients, 2566 were enrolled in 12 double-blind placebo-controlled studies; 591 (23%) were 65 years and over, while 82 (3%) were 75 years and over. No overall differences in safety or effectiveness were observed between patients 65 years and over and younger patients. Therefore, no dose adjustment is recommended in the elderly population. While clinical studies of linagliptin have not identified differences in response between the elderly and younger patients, greater sensitivity of some older individuals cannot be ruled out.
Controlled clinical studies of metformin did not include sufficient numbers of elderly patients to determine whether they respond differently from younger patients, although other reported clinical experience has not identified differences in responses between the elderly and young patients. The initial and maintenance dosing of metformin should be conservative in patients with advanced age, due to the potential for decreased renal function in this population. Any dose adjustment should be based on a careful assessment of renal function [see Contraindications (4), Warnings and Precautions (5.3), and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
In the event of an overdose with JENTADUETO, contact the Poison Control Center. Employ the usual supportive measures (e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive treatment) as dictated by the patient’s clinical status. Removal of linagliptin by hemodialysis or peritoneal dialysis is unlikely. However, metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful partly for removal of accumulated metformin from patients in whom JENTADUETO overdosage is suspected.
During controlled clinical trials in healthy subjects, with single doses of up to 600 mg of linagliptin (equivalent to 120 times the recommended daily dose), there were no dose-related clinical adverse drug reactions. There is no experience with doses above 600 mg in humans.
Overdose of metformin has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [see Boxed Warning and Warnings and Precautions (5.1)].
11 DESCRIPTION
JENTADUETO tablets contain 2 oral antihyperglycemic drugs used in the management of type 2 diabetes mellitus: linagliptin and metformin hydrochloride.
Linagliptin is an orally-active inhibitor of the dipeptidyl peptidase-4 (DPP-4) enzyme.
Linagliptin is described chemically as 1H-Purine-2,6-dione, 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-
The empirical formula is C25H28N8O2 and the molecular weight is 472.54 g/mol. The structural formula is:
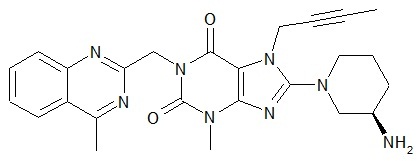
Linagliptin is a white to yellowish, not or only slightly hygroscopic solid substance. It is very slightly soluble in water (0.9 mg/mL). Linagliptin is soluble in methanol (ca. 60 mg/mL), sparingly soluble in ethanol (ca. 10 mg/mL), very slightly soluble in isopropanol (<1 mg/mL), and very slightly soluble in acetone (ca. 1 mg/mL).
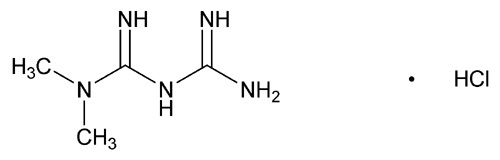
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is not chemically or pharmacologically related to any other classes of oral antihyperglycemic agents. Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5•HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is:
JENTADUETO is available for oral administration as tablets containing 2.5 mg linagliptin and 500 mg metformin hydrochloride (JENTADUETO 2.5 mg/500 mg), 850 mg metformin hydrochloride (JENTADUETO 2.5 mg/850 mg) or 1000 mg metformin hydrochloride (JENTADUETO 2.5 mg/1000 mg). Each film-coated tablet of JENTADUETO contains the following inactive ingredients: arginine, corn starch, copovidone, colloidal silicon dioxide, magnesium stearate, titanium dioxide, propylene glycol, hypromellose, talc, yellow ferric oxide (2.5 mg/500 mg; 2.5 mg/850 mg) and/or red ferric oxide (2.5 mg/850 mg; 2.5 mg/1000 mg).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
JENTADUETO combines 2 antihyperglycemic agents with complementary mechanisms of action to improve glycemic control in patients with type 2 diabetes mellitus: linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, and metformin, a member of the biguanide class.
Linagliptin is an inhibitor of DPP-4, an enzyme that degrades the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Thus, linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin in a glucose-dependent manner and decreasing the levels of glucagon in the circulation. Both incretin hormones are involved in the physiological regulation of glucose homeostasis. Incretin hormones are secreted at a low basal level throughout the day and levels rise immediately after meal intake. GLP-1 and GIP increase insulin biosynthesis and secretion from pancreatic beta cells in the presence of normal and elevated blood glucose levels. Furthermore, GLP-1 also reduces glucagon secretion from pancreatic alpha cells, resulting in a reduction in hepatic glucose output.
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. Its pharmacologic mechanisms of action are different from other classes of oral antihyperglycemic agents. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike SUs, metformin does not produce hypoglycemia in either patients with type 2 diabetes mellitus or normal subjects (except in special circumstances) [see Warnings and Precautions (5.9)] and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
12.2 Pharmacodynamics
Linagliptin binds to DPP-4 in a reversible manner and increases the concentrations of incretin hormones. Linagliptin glucose-dependently increases insulin secretion and lowers glucagon secretion, thus resulting in a better regulation of the glucose homeostasis. Linagliptin binds selectively to DPP-4 and selectively inhibits DPP-4, but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures.
In a randomized, placebo-controlled, active-comparator, 4-way crossover study, 36 healthy subjects were administered a single oral dose of linagliptin 5 mg, linagliptin 100 mg (20 times the recommended dose), moxifloxacin, and placebo. No increase in QTc was observed with either the recommended dose of 5 mg or the 100-mg dose. At the 100-mg dose, peak linagliptin plasma concentrations were approximately 38-fold higher than the peak concentrations following a 5-mg dose.
12.3 Pharmacokinetics
The results of a bioequivalence study in healthy subjects demonstrated that JENTADUETO (linagliptin/metformin hydrochloride) 2.5 mg/500 mg, 2.5 mg/850 mg, and 2.5 mg/1000 mg combination tablets are bioequivalent to coadministration of corresponding doses of linagliptin and metformin as individual tablets. Administration of linagliptin 2.5 mg/metformin hydrochloride 1000 mg fixed-dose combination with food resulted in no change in overall exposure of linagliptin. There was no change in metformin AUC; however, mean peak serum concentration of metformin was decreased by 18% when administered with food. A delayed time-to-peak serum concentrations by 2 hours was observed for metformin under fed conditions. These changes are not likely to be clinically significant.
The absolute bioavailability of linagliptin is approximately 30%. Following oral administration, plasma concentrations of linagliptin decline in at least a biphasic manner with a long terminal half-life (>100 hours), related to the saturable binding of linagliptin to DPP-4. However, the prolonged elimination does not contribute to the accumulation of the drug. The effective half-life for accumulation of linagliptin, as determined from oral administration of multiple doses of linagliptin 5 mg, is approximately 12 hours. After once-daily dosing, steady state plasma concentrations of linagliptin 5 mg are reached by the third dose, and Cmax and AUC increased by a factor of 1.3 at steady-state compared with the first dose. Plasma AUC of linagliptin increased in a less than dose-proportional manner in the dose range of 1 to 10 mg. The pharmacokinetics of linagliptin is similar in healthy subjects and in patients with type 2 diabetes.
The absolute bioavailability of a metformin hydrochloride 500-mg tablet given under fasting conditions is approximately 50% to 60%. Studies using single oral doses of metformin tablets 500 mg to 1500 mg, and 850 mg to 2550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination.
The mean apparent volume of distribution at steady state following a single intravenous dose of linagliptin 5 mg to healthy subjects is approximately 1110 L, indicating that linagliptin extensively distributes to the tissues. Plasma protein binding of linagliptin is concentration-dependent decreasing from about 99% at 1 nmol/L to 75% to 89% at ≥30 nmol/L, reflecting saturation of binding to DPP-4 with increasing concentration of linagliptin. At high concentrations, where DPP-4 is fully saturated, 70% to 80% of linagliptin remains bound to plasma proteins and 20% to 30% is unbound in plasma. Plasma binding is not altered in patients with renal or hepatic impairment.
The apparent volume of distribution (V/F) of metformin following single oral doses of immediate-release metformin hydrochloride tablets 850 mg averaged 654±358 L. Metformin is negligibly bound to plasma proteins, in contrast to SUs, which are more than 90% protein bound. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin tablets, steady-state plasma concentrations of metformin are reached within 24 to 48 hours and are generally <1 mcg/mL. During controlled clinical trials of metformin, maximum metformin plasma levels did not exceed 5 mcg/mL, even at maximum doses.
Following oral administration, the majority (about 90%) of linagliptin is excreted unchanged, indicating that metabolism represents a minor elimination pathway. A small fraction of absorbed linagliptin is metabolized to a pharmacologically inactive metabolite, which shows a steady-state exposure of 13.3% relative to linagliptin.
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion.
Following administration of an oral [14C]linagliptin dose to healthy subjects, approximately 85% of the administered radioactivity was eliminated via the enterohepatic system (80%) or urine (5%) within 4 days of dosing. Renal clearance at steady state was approximately 70 mL/min.
Renal clearance is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
JENTADUETO: Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in renally impaired patients have not been performed. Since metformin is contraindicated in patients with renal impairment, use of JENTADUETO is also contraindicated in patients with renal impairment (e.g., serum creatinine ≥1.5 mg/dL [males] or ≥1.4 mg/dL [females], or abnormal creatinine clearance) [see Contraindications (4) and Warnings and Precautions (5.3)].
Linagliptin: Under steady-state conditions, linagliptin exposure in patients with mild renal impairment was comparable to healthy subjects. In patients with moderate renal impairment under steady-state conditions, mean exposure of linagliptin increased (AUCτ,ss by 71% and Cmax by 46%) compared with healthy subjects. This increase was not associated with a prolonged accumulation half-life, terminal half-life, or an increased accumulation factor. Renal excretion of linagliptin was below 5% of the administered dose and was not affected by decreased renal function.
Patients with type 2 diabetes mellitus and severe renal impairment showed steady-state exposure approximately 40% higher than that of patients with type 2 diabetes mellitus and normal renal function (increase in AUC by 42% and Cmax by 35%). For both type 2 diabetes mellitus groups, renal excretion was below 7% of the administered dose.
Metformin: In patients with decreased renal function (based on measured creatinine clearance), the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased in proportion to the decrease in creatinine clearance [see Contraindications (4) and Warnings and Precautions (5.3)].
JENTADUETO: Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in hepatically impaired patients have not been performed. However, use of metformin alone in patients with hepatic impairment has been associated with some cases of lactic acidosis. Therefore, use of JENTADUETO is not recommended in patients with hepatic impairment [see Warnings and Precautions (5.4)].
Linagliptin: In patients with mild hepatic impairment (Child-Pugh class A) steady-state exposure (AUCτ,ss) of linagliptin was approximately 25% lower and Cmax,ss was approximately 36% lower than in healthy subjects. In patients with moderate hepatic impairment (Child-Pugh class B), AUCss, of linagliptin was about 14% lower and Cmax,ss was approximately 8% lower than in healthy subjects. Patients with severe hepatic impairment (Child-Pugh class C) had comparable exposure of linagliptin in terms of AUC0-24 and approximately 23% lower Cmax compared with healthy subjects. Reductions in the pharmacokinetic parameters seen in patients with hepatic impairment did not result in reductions in DPP-4 inhibition.
Metformin hydrochloride: No pharmacokinetic studies of metformin have been conducted in patients with hepatic impairment.
Linagliptin: BMI/Weight had no clinically meaningful effect on the pharmacokinetics of linagliptin based on a population pharmacokinetic analysis.
Linagliptin: Gender had no clinically meaningful effect on the pharmacokinetics of linagliptin based on a population pharmacokinetic analysis.
Metformin hydrochloride: Metformin pharmacokinetic parameters did not differ significantly between normal subjects and patients with type 2 diabetes mellitus when analyzed according to gender. Similarly, in controlled clinical studies in patients with type 2 diabetes mellitus, the antihyperglycemic effect of metformin was comparable in males and females.
JENTADUETO: Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in geriatric patients have not been performed. Based on the metformin component, JENTADUETO treatment should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced [see Warnings and Precautions (5.1, 5.3) and Use in Specific Populations (8.5)].
Linagliptin: Age did not have a clinically meaningful impact on the pharmacokinetics of linagliptin based on a population pharmacokinetic analysis.
Metformin hydrochloride: Limited data from controlled pharmacokinetic studies of metformin in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and Cmax is increased, compared with healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function.
Studies characterizing the pharmacokinetics of linagliptin and metformin after administration of JENTADUETO in pediatric patients have not yet been performed.
Linagliptin: Race had no clinically meaningful effect on the pharmacokinetics of linagliptin based on available pharmacokinetic data, including subjects of White, Hispanic, Black, and Asian racial groups.
Metformin hydrochloride: No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin in patients with type 2 diabetes mellitus, the antihyperglycemic effect was comparable in Caucasians (n=249), Blacks (n=51), and Hispanics (n=24).
Pharmacokinetic drug interaction studies with JENTADUETO have not been performed; however, such studies have been conducted with the individual components of JENTADUETO (linagliptin and metformin hydrochloride).
In vitro Assessment of Drug Interactions
Linagliptin is a weak to moderate inhibitor of CYP isozyme CYP3A4, but does not inhibit other CYP isozymes and is not an inducer of CYP isozymes, including CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 4A11.
Linagliptin is a P-glycoprotein (P-gp) substrate, and inhibits P-gp mediated transport of digoxin at high concentrations. Based on these results and in vivo drug interaction studies, linagliptin is considered unlikely to cause interactions with other P-gp substrates at therapeutic concentrations.
In vivo Assessment of Drug Interactions
Inducers of CYP3A4 or P-gp (e.g., rifampin) decrease exposure to linagliptin to subtherapeutic and likely ineffective concentrations. For patients requiring use of such drugs, an alternative to linagliptin is strongly recommended. In vivo studies indicated evidence of a low propensity for causing drug interactions with substrates of CYP3A4, CYP2C9, CYP2C8, P-gp, and OCT. No dose adjustment of linagliptin is recommended based on results of the described pharmacokinetic studies.
| *Multiple dose (steady state) unless otherwise noted # Single dose †AUC = AUC(0 to 24 hours) for single-dose treatments and AUC = AUC(TAU) for multiple-dose treatments QD = once daily BID = twice daily TID = three times daily |
||||
| Coadministered Drug | Dosing of Coadministered Drug* | Dosing of Linagliptin* | Geometric Mean Ratio (ratio with/without coadministered drug) No effect=1.0 |
|
| AUC† | Cmax | |||
| No dosing adjustments required for linagliptin when given with the following coadministered drugs: | ||||
| Metformin | 850 mg TID | 10 mg QD | 1.20 | 1.03 |
| Glyburide | 1.75 mg# | 5 mg QD | 1.02 | 1.01 |
| Pioglitazone | 45 mg QD | 10 mg QD | 1.13 | 1.07 |
| Ritonavir | 200 mg BID | 5 mg# | 2.01 | 2.96 |
| The efficacy of JENTADUETO may be reduced when administered in combination with strong inducers of CYP3A4 or P-gp (e.g., rifampin). Use of alternative treatments is strongly recommended [see Drug Interactions (7.2)]. | ||||
| Rifampin | 600 mg QD | 5 mg QD | 0.60 | 0.56 |
| * Multiple dose (steady state) unless otherwise noted # Single dose †AUC = AUC(INF) for single-dose treatments and AUC = AUC(TAU) for multiple-dose treatments **AUC=AUC(0-168) and Cmax=Emax for pharmacodynamic end points INR = International Normalized Ratio PT = Prothrombin Time QD = once daily TID = three times daily |
|||||
| Coadministered Drug | Dosing of Coadministered Drug* | Dosing of Linagliptin* | Geometric Mean Ratio (ratio with/without coadministered drug) No effect=1.0 |
||
| AUC† | Cmax | ||||
| No dosing adjustments required for the following coadministered drugs: | |||||
| Metformin | 850 mg TID | 10 mg QD | metformin | 1.01 | 0.89 |
| Glyburide | 1.75 mg# | 5 mg QD | glyburide | 0.86 | 0.86 |
| Pioglitazone | 45 mg QD | 10 mg QD | pioglitazone metabolite M-III metabolite M-IV | 0.94 0.98 1.04 | 0.86 0.96 1.05 |
| Digoxin | 0.25 mg QD | 5 mg QD | digoxin | 1.02 | 0.94 |
| Simvastatin | 40 mg QD | 10 mg QD | simvastatin simvastatin acid | 1.34 1.33 | 1.10 1.21 |
| Warfarin | 10 mg# | 5 mg QD | R-warfarin S-warfarin INR PT | 0.99 1.03 0.93** 1.03** | 1.00 1.01 1.04** 1.15** |
| Ethinylestradiol and levonorgestrel | ethinylestradiol 0.03 mg and levonorgestrel 0.150 mg QD | 5 mg QD | ethinylestradiol levonorgestrel | 1.01 1.09 | 1.08 1.13 |
| * All metformin and coadministered drugs were given as single doses † AUC = AUC(INF) ‡ Ratio of arithmetic means **At steady state with topiramate 100 mg every 12 hours and metformin 500 mg every 12 hours; AUC = AUC0-12h |
|||||
| Coadministered Drug | Dosing of Coadministered Drug* | Dose of Metformin* | Geometric Mean Ratio (ratio with/without coadministered drug) No effect=1.0 |
||
| AUC† | Cmax | ||||
| No dosing adjustments required for the following coadministered drugs: | |||||
| Furosemide | 40 mg | 850 mg | metformin | 1.09‡ | 1.22‡ |
| Nifedipine | 10 mg | 850 mg | metformin | 1.16 | 1.21 |
| Propranolol | 40 mg | 850 mg | metformin | 0.90 | 0.94 |
| Ibuprofen | 400 mg | 850 mg | metformin | 1.05‡ | 1.07‡ |
| Cationic drugs eliminated by renal tubular secretion may reduce metformin elimination: use with caution [see Warnings and Precautions (5.3) and Drug Interactions (7.1)]. | |||||
| Cimetidine | 400 mg | 850 mg | metformin | 1.40 | 1.61 |
| Carbonic anhydrase inhibitors may cause metabolic acidosis: use with caution [see Warnings and Precautions (5.1) and Drug Interactions (7.1)]. | |||||
| Topiramate** | 100 mg | 500 mg | metformin | 1.25 | 1.17 |
| * All metformin and coadministered drugs were given as single doses † AUC = AUC(INF) unless otherwise noted ‡ Ratio of arithmetic means, p-value of difference <0.05 § AUC(0-24 hr) reported ¶ Ratio of arithmetic means |
|||||
| Coadministered Drug | Dosing of Coadministered Drug* | Dose of Metformin* | Geometric Mean Ratio (ratio with/without metformin) No effect=1.0 |
||
| AUC† | Cmax | ||||
| No dosing adjustments required for the following coadministered drugs: | |||||
| Glyburide | 5 mg | 500 mg§ | glyburide | 0.78‡ | 0.63‡ |
| Furosemide | 40 mg | 850 mg | furosemide | 0.87‡ | 0.69‡ |
| Nifedipine | 10 mg | 850 mg | nifedipine | 1.10§ | 1.08 |
| Propranolol | 40 mg | 850 mg | propranolol | 1.01§ | 0.94 |
| Ibuprofen | 400 mg | 850 mg | ibuprofen | 0.97¶ | 1.01¶ |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
JENTADUETO
No animal studies have been conducted with the combined products in JENTADUETO to evaluate carcinogenesis, mutagenesis, or impairment of fertility. General toxicity studies in rats up to 13 weeks were performed with JENTADUETO.
The following data are based on the findings in studies with linagliptin and metformin individually.
Linagliptin
Linagliptin did not increase the incidence of tumors in male and female rats in a 2-year study at doses of 6, 18, and 60 mg/kg. The highest dose of 60 mg/kg is approximately 418 times the clinical dose of 5 mg/day based on AUC exposure. Linagliptin did not increase the incidence of tumors in mice in a 2-year study at doses up to 80 mg/kg (males) and 25 mg/kg (females), or approximately 35 and 270 times the clinical dose based on AUC exposure. Higher doses of linagliptin in female mice (80 mg/kg) increased the incidence of lymphoma at approximately 215 times the clinical dose based on AUC exposure.
Linagliptin was not mutagenic or clastogenic with or without metabolic activation in the Ames bacterial mutagenicity assay, a chromosomal aberration test in human lymphocytes, and an in vivo micronucleus assay.
In fertility studies in rats, linagliptin had no adverse effects on early embryonic development, mating, fertility, or bearing live young up to the highest dose of 240 mg/kg (approximately 943 times the clinical dose based on AUC exposure).
Metformin Hydrochloride
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1500 mg/kg/day, respectively. These doses are both approximately 4 times the maximum recommended human daily dose of 2000 mg/kg/day based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of a mutagenic potential of metformin in the following in vitro tests: Ames test (Salmonella typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin when administered at doses as high as 600 mg/kg/day, which is approximately 2 times the MRHD based on body surface area comparisons.
14 CLINICAL STUDIES
The coadministration of linagliptin and metformin has been studied in patients with type 2 diabetes mellitus inadequately controlled on diet and exercise and in combination with sulfonylurea.
There have been no clinical efficacy studies conducted with JENTADUETO; however, bioequivalence of JENTADUETO to linagliptin and metformin coadministered as individual tablets was demonstrated in healthy subjects.
14.1 Initial Combination Therapy with Metformin
A total of 791 patients with type 2 diabetes mellitus and inadequate glycemic control on diet and exercise participated in the 24-week, randomized, double-blind, portion of this placebo-controlled factorial study designed to assess the efficacy of linagliptin as initial therapy with metformin. Patients on an antihyperglycemic agent (52%) underwent a drug washout period of 4 weeks’ duration. After the washout period and after completing a 2-week single-blind placebo run-in period, patients with inadequate glycemic control (A1C ≥7.0% to ≤10.5%) were randomized. Patients with inadequate glycemic control (A1C ≥7.5% to <11.0%) not on antihyperglycemic agents at study entry (48%) immediately entered the 2-week single-blind placebo run-in period and then were randomized. Randomization was stratified by baseline A1C (<8.5% vs ≥8.5%) and use of a prior oral antidiabetic drug (none vs monotherapy). Patients were randomized in a 1:2:2:2:2:2 ratio to either placebo or one of 5 active-treatment arms. Approximately equal numbers of patients were randomized to receive initial therapy with 5 mg of linagliptin once daily, 500 mg or 1000 mg of metformin twice daily, or 2.5 mg of linagliptin twice daily in combination with 500 mg or 1000 mg of metformin twice daily. Patients who failed to meet specific glycemic goals during the study were treated with sulfonylurea, thiazolidinedione, or insulin rescue therapy.
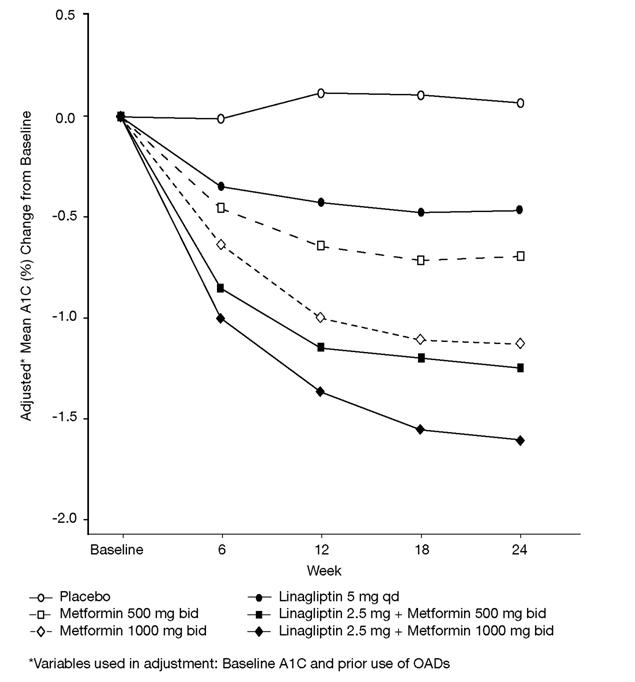
Initial therapy with the combination of linagliptin and metformin provided significant improvements in A1C, and fasting plasma glucose (FPG) compared to placebo, to metformin alone, and to linagliptin alone (Table 6, Figure 1). The adjusted mean treatment difference in A1C from baseline to week 24 (LOCF) was -0.5% (95% CI -0.7, -0.3; p<0.0001) for linagliptin 2.5 mg/metformin 1000 mg twice daily compared to metformin 1000 mg twice daily; -1.1% (95% CI -1.4, -0.9; p<0.0001) for linagliptin 2.5 mg/metformin 1000 mg twice daily compared to linagliptin 5 mg once daily; -0.6% (95% CI -0.8, -0.4; p<0.0001) for linagliptin 2.5 mg/metformin 500 mg twice daily compared to metformin 500 mg twice daily; and -0.8% (95% CI -1.0, -0.6; p<0.0001) for linagliptin 2.5 mg/metformin 500 mg twice daily compared to linagliptin 5 mg once daily.
Lipid effects were generally neutral. No meaningful change in body weight was noted in any of the 6 treatment groups.
| *Total daily dose of linagliptin is equal to 5 mg **Full analysis population using last observation on study ***Metformin 500 mg twice daily, n=140; Linagliptin 2.5 mg twice daily + Metformin 500 twice daily, n=136; Metformin 1000 mg twice daily, n=137; Linagliptin 2.5 mg twice daily + Metformin 1000 mg twice daily, n=138. ****HbA1c: ANCOVA model included treatment and number of prior OADs as class-effects, as well as baseline HbA1c as continuous covariates. FPG: ANCOVA model included treatment and number of prior OADs as class-effects, as well as baseline HbA1c and baseline FPG as continuous covariates. |
||||||
| Placebo | Linagliptin 5 mg Once Daily* | Metformin 500 mg Twice Daily | Linagliptin 2.5 mg Twice Daily* + Metformin 500 mg Twice Daily | Metformin 1000 mg Twice Daily | Linagliptin 2.5 mg Twice Daily* + Metformin 1000 mg Twice Daily |
|
| A1C (%) | ||||||
| Number of patients | n=65 | n=135 | n=141 | n=137 | n=138 | n=140 |
| Baseline (mean) | 8.7 | 8.7 | 8.7 | 8.7 | 8.5 | 8.7 |
| Change from baseline (adjusted mean****) | 0.1 | -0.5 | -0.6 | -1.2 | -1.1 | -1.6 |
| Difference from placebo (adjusted mean) (95% CI) | -- | -0.6 (-0.9, -0.3) | -0.8 (-1.0, -0.5) | -1.3 (-1.6, -1.1) | -1.2 (-1.5, -0.9) | -1.7 (-2.0, -1.4) |
| Patients [n (%)] achieving A1C <7%*** | 7 (10.8) | 14 (10.4) | 26 (18.6) | 41 (30.1) | 42 (30.7) | 74 (53.6) |
| Patients (%) receiving rescue medication | 29.2 | 11.1 | 13.5 | 7.3 | 8.0 | 4.3 |
| FPG (mg/dL) | ||||||
| Number of patients | n=61 | n=134 | n=136 | n=135 | n=132 | n=136 |
| Baseline (mean) | 203 | 195 | 191 | 199 | 191 | 196 |
| Change from baseline (adjusted mean****) | 10 | -9 | -16 | -33 | -32 | -49 |
| Difference from placebo (adjusted mean) (95% CI) | -- | -19 (-31, -6) | -26 (-38, -14) | -43 (-56, -31) | -42 (-55, -30) | -60 (-72, -47) |
Figure 1 Adjusted Mean Change from Baseline for A1C (%) over 24 Weeks with Linagliptin and Metformin, Alone and in Combination in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Diet and Exercise - FAS completers
14.2 Add-On Combination Therapy with Metformin
A total of 701 patients with type 2 diabetes participated in a 24-week, randomized, double-blind, placebo-controlled study designed to assess the efficacy of linagliptin in combination with metformin. Patients already on metformin (n=491) at a dose of at least 1500 mg per day were randomized after completing a 2-week open-label placebo run-in period. Patients on metformin and another antihyperglycemic agent (n=207) were randomized after a run-in period of approximately 6 weeks on metformin (at a dose of at least 1500 mg per day) in monotherapy. Patients were randomized to the addition of either linagliptin 5 mg or placebo, administered once daily. Patients who failed to meet specific glycemic goals during the studies were treated with glimepiride rescue.
In combination with metformin, linagliptin provided statistically significant improvements in A1C, FPG, and 2-hour PPG compared with placebo (Table 7). Rescue glycemic therapy was used in 7.8% of patients treated with linagliptin 5 mg and in 18.9% of patients treated with placebo. A similar decrease in body weight was observed for both treatment groups.
| * Full analysis population using last observation on study **Linagliptin 5 mg + Metformin, n=485; Placebo + Metformin, n=163. ***HbA1c: ANCOVA model included treatment and number of prior oral OADs as class-effects, as well as baseline HbA1c as continuous covariates. FPG: ANCOVA model included treatment and number of prior OADs as class-effects, as well as baseline HbA1c and baseline FPG as continuous covariates. PPG: ANCOVA model included treatment and number of prior OADs as class-effects, as well as baseline HbA1c and baseline postprandial glucose after two hours as covariate. |
||
| Linagliptin 5 mg + Metformin | Placebo + Metformin | |
| A1C (%) | ||
| Number of patients | n=513 | n=175 |
| Baseline (mean) | 8.1 | 8.0 |
| Change from baseline (adjusted mean***) | -0.5 | 0.15 |
| Difference from placebo + metformin (adjusted mean) (95% CI) | -0.6 (-0.8, -0.5) | -- |
| Patients [n (%)] achieving A1C <7% ** | 127 (26.2) | 15 (9.2) |
| FPG (mg/dL) | ||
| Number of patients | n=495 | n=159 |
| Baseline (mean) | 169 | 164 |
| Change from baseline (adjusted mean***) | -11 | 11 |
| Difference from placebo + metformin (adjusted mean) (95% CI) | -21 (-27, -15) | -- |
| 2-hour PPG (mg/dL) | ||
| Number of patients | n=78 | n=21 |
| Baseline (mean) | 270 | 274 |
| Change from baseline (adjusted mean***) | -49 | 18 |
| Difference from placebo + metformin (adjusted mean) (95% CI) | -67 (-95, -40) | -- |
14.3 Active-Controlled Study vs Glimepiride in Combination with Metformin
The efficacy of linagliptin was evaluated in a 104-week double-blind, glimepiride-controlled non-inferiority study in type 2 diabetic patients with insufficient glycemic control despite metformin therapy. Patients being treated with metformin only entered a run-in period of 2 weeks’ duration, whereas patients pretreated with metformin and one additional antihyperglycemic agent entered a run-in treatment period of 6 weeks’ duration with metformin monotherapy (dose of ≥1500 mg per day) and washout of the other agent. After an additional 2-week placebo run-in period, those with inadequate glycemic control (A1C 6.5% to 10%) were randomized 1:1 to the addition of linagliptin 5 mg once daily or glimepiride. Randomization was stratified by baseline HbA1c (<8.5% vs ≥8.5%), and the previous use of antidiabetic drugs (metformin alone vs metformin plus one other OAD). Patients receiving glimepiride were given an initial dose of 1 mg/day and then electively titrated over the next 12 weeks to a maximum dose of 4 mg/day as needed to optimize glycemic control. Thereafter, the glimepiride dose was to be kept constant, except for down-titration to prevent hypoglycemia.
After 52 weeks and 104 weeks, linagliptin and glimepiride both had reductions from baseline in A1C (52 weeks: -0.4% for linagliptin, -0.6% for glimepiride; 104 weeks: -0.2% for TRADJENTA, -0.4% for glimepiride) from a baseline mean of 7.7% (Table 8). The mean difference between groups in A1C change from baseline was 0.2% with 2-sided 97.5% confidence interval (0.1%, 0.3%) for the intent-to-treat population using last observation carried forward. These results were consistent with the completers analysis.
| *p<0.0001 vs glimepiride; †p=0.0012 vs glimepiride **Full analysis population using last observation on study ***Hypoglycemic incidence included both asymptomatic events (not accompanied by typical symptoms and plasma glucose concentration of ≤70 mg/dL) and symptomatic events with typical symptoms of hypoglycemia and plasma glucose concentration of ≤70 mg/dL. ****HbA1c: ANCOVA model included treatment and number of prior OADs as class-effects, as well as baseline HbA1c as continuous covariates. FPG: ANCOVA model included treatment and number of prior OADs as class-effects, as well as baseline HbA1c and baseline FPG as continuous covariates. Hypoglycemia incidence (%): Cochran-Mantel-Haenszel test was performed on the patient population contained in the treated set, to compare the proportion of patients with hypoglycemic events between patients treated with linagliptin and patients treated with glimepiride. | ||||
| Week 52 | Week 104 | |||
| Linagliptin 5 mg + Metformin | Glimepiride + Metformin (mean glimepiride dose 3 mg) | TRADJENTA 5 mg + Metformin | Glimepiride + Metformin (mean glimepiride dose 3 mg) |
|
| A1C (%) | ||||
| Number of patients | n=764 | n=755 | n=764 | n=755 |
| Baseline (mean) | 7.7 | 7.7 | 7.7 | 7.7 |
| Change from baseline (adjusted mean****) | -0.4 | -0.6 | -0.2 | -0.4 |
| Difference from glimepiride (adjusted mean) (97.5% CI) | 0.2 (0.1, 0.3) | -- | 0.2 (0.1, 0.3) | -- |
| FPG (mg/dL) | ||||
| Number of patients | n=733 | n=725 | n=733 | n=725 |
| Baseline (mean) | 164 | 166 | 164 | 166 |
| Change from baseline (adjusted mean****) | 8* | -15 | -2† | -9 |
| Hypoglycemia incidence (%)*** | ||||
| Number of patients | n=776 | n=775 | n=776 | n=775 |
| Incidence | 5.3* | 31.1 | 7.5 * | 36.1 |
Patients treated with linagliptin had a mean baseline body weight of 86 kg and were observed to have an adjusted mean decrease in body weight of 1.1 kg at 52 weeks and 1.4 kg at 104 weeks. Patients on glimepiride had a mean baseline body weight of 87 kg and were observed to have an adjusted mean increase from baseline in body weight of 1.4 kg at 52 weeks and 1.3 kg at 104 weeks (treatment difference p<0.0001 for both timepoints).
14.4 Add-On Combination Therapy with Metformin and a Sulfonylurea
A total of 1058 patients with type 2 diabetes mellitus participated in a 24-week, randomized, double-blind, placebo-controlled study designed to assess the efficacy of linagliptin in combination with a sulfonylurea and metformin. The most common sulfonylureas used by patients in the study were glimepiride (31%), glibenclamide (26%), and gliclazide (26% [not available in the United States]). Patients on a sulfonylurea and metformin were randomized to receive linagliptin 5 mg or placebo, each administered once daily. Patients who failed to meet specific glycemic goals during the study were treated with pioglitazone rescue. Glycemic end points measured included A1C and FPG.
In combination with a sulfonylurea and metformin, linagliptin provided statistically significant improvements in A1C and FPG compared with placebo (Table 9). In the entire study population (patients on linagliptin in combination with a sulfonylurea and metformin), a mean reduction from baseline relative to placebo in A1C of -0.6% and in FPG of -13 mg/dL was seen. Rescue therapy was used in 5.4% of patients treated with linagliptin 5 mg and in 13% of patients treated with placebo. Change from baseline in body weight did not differ significantly between the groups.
| SU=sulfonylurea *Full analysis population using last observation on study **Linagliptin 5 mg + Metformin + SU, n=742; Placebo + Metformin + SU, n=247 ***HbA1c: ANCOVA model included treatment as class-effects and baseline HbA1c as continuous covariates. FPG: ANCOVA model included treatment as class-effects, as well as baseline HbA1c and baseline FPG as continuous covariates. |
||
| Linagliptin 5 mg + Metformin + SU | Placebo + Metformin + SU | |
| A1C (%) | ||
| Number of patients | n=778 | n=262 |
| Baseline (mean) | 8.2 | 8.1 |
| Change from baseline (adjusted mean***) | -0.7 | -0.1 |
| Difference from placebo (adjusted mean) (95% CI) | -0.6 (-0.7, -0.5) | -- |
| Patients [n (%)] achieving A1C <7%** | 217 (29.2) | 20 (8.1) |
| FPG (mg/dL) | ||
| Number of patients | n=739 | n=248 |
| Baseline (mean) | 159 | 163 |
| Change from baseline (adjusted mean***) | -5 | 8 |
| Difference from placebo (adjusted mean) (95% CI) | -13 (-18, -7) | -- |
14.5 Add-On Combination Therapy with Insulin
A total of 1261 patients with type 2 diabetes inadequately controlled on basal insulin alone or basal insulin in combination with oral drugs participated in a randomized, double-blind placebo-controlled trial designed to evaluate the efficacy of linagliptin as add-on therapy to basal insulin over 24 weeks. Randomization was stratified by baseline HbA1c (<8.5% vs ≥8.5%), renal function impairment status (based on baseline eGFR), and concomitant use of oral antidiabetic drugs (none, metformin only, pioglitazone only, metformin + pioglitazone). Patients with a baseline A1C of >7% and <10% were included in the study including 709 patients with renal impairment (eGFR <90 mL/min), most of whom (n=575) were categorized as mild renal impairment (eGFR 60 to <90 mL/min). Patients entered a 2-week placebo run-in period on basal insulin (e.g., insulin glargine, insulin detemir, or NPH insulin) with or without metformin and/or pioglitazone background therapy. Following the run-in period, patients with inadequate glycemic control were randomized to the addition of either 5 mg of linagliptin or placebo, administered once daily. Patients were maintained on a stable dose of insulin prior to enrollment, during the run-in period, and during the first 24 weeks of treatment. Patients who failed to meet specific glycemic goals during the double-blind treatment period were rescued by increasing background insulin dose.
Linagliptin used in combination with insulin (with or without metformin and/or pioglitazone), provided statistically significant improvements in A1C and FPG compared to placebo (Table 10) after 24 weeks of treatment. The mean total daily insulin dose at baseline was 42 units for patients treated with linagliptin and 40 units for patients treated with placebo. Background baseline diabetes therapy included use of: insulin alone (16.1%), insulin combined with metformin only (75.5%), insulin combined with metformin and pioglitazone (7.4%), and insulin combined with pioglitazone only (1%). The mean change from baseline to Week 24 in the daily dose of insulin was +1.3 IU in the placebo group and +0.6 IU in the linagliptin group. The mean change in body weight from baseline to Week 24 was similar in the two treatment groups. The rate of hypoglycemia, defined as all symptomatic or asymptomatic episodes with a self measured blood glucose was also similar in both groups (21.4% linagliptin; 22.9% placebo) in the first 24 weeks of the study.
| *Full analysis population using last observation carried forward (LOCF) method on study **Linagliptin + Insulin, n=595; Placebo + Insulin, n=593 ***HbA1c: ANCOVA model included treatment, categorical renal function impairment status and concomitant OADs as class-effects, as well as baseline HbA1c as continuous covariates. FPG: ANCOVA model included treatment, categorical renal function impairment status and concomitant OADs as class-effects, as well as baseline HbA1c and baseline FPG as continuous covariates. |
||
| Linagliptin 5 mg + Insulin | Placebo + Insulin | |
| A1C (%) | ||
| Number of patients | n=618 | n=617 |
| Baseline (mean) | 8.3 | 8.3 |
| Change from baseline (adjusted mean***) | -0.6 | 0.1 |
| Difference from placebo (adjusted mean) (95% CI) | -0.7 (-0.7, -0.6) | -- |
| Patients [n (%)] achieving A1C <7%** | 116 (19.5) | 48 (8.1) |
| FPG (mg/dL) | ||
| Number of patients | n=613 | n=608 |
| Baseline (mean) | 147 | 151 |
| Change from baseline (adjusted mean***) | -8 | 3 |
| Difference from placebo (adjusted mean) (95% CI) | -11 (-16, -6) | -- |
The difference between treatment with linagliptin and placebo in terms of adjusted mean change from baseline in HbA1c after 24 weeks was comparable for patients with no renal impairment (eGRF ≥90 mL/min, n=539), with mild renal impairment (eGFR 60 to <90 mL/min, n=565), or with moderate renal impairment (eGFR 30 to <60 mL/min, n=124).
14.6 Renal Impairment
A total of 133 patients with type 2 diabetes participated in a 52 week, double-blind, randomized, placebo-controlled trial designed to evaluate the efficacy and safety of linagliptin in patients with both type 2 diabetes and severe chronic renal impairment. Participants with an estimated (based on the four variables modified diet in renal disease [MDRD] equation) GFR value of <30 mL/min were eligible to participate in the study. Randomization was stratified by baseline HbA1c (≤8% and >8%) and background antidiabetic therapy (insulin or any combination with insulin, SU or glinides as monotherapy and pioglitazone or any other antidiabetics excluding any other DPP-4 inhibitors). For the initial 12 weeks of the study, background antidiabetic therapy was kept stable and included insulin, sulfonylurea, glinides, and pioglitazone. For the remainder of the trial, dose adjustments in antidiabetic background therapy were allowed. At baseline in this trial, 62.5% of patients were receiving insulin alone as background diabetes therapy, and 12.5% were receiving sulfonylurea alone.
After 12 weeks of treatment, linagliptin 5 mg provided statistically significant improvement in A1C compared to placebo, with an adjusted mean change of -0.6% compared to placebo (95% confidence interval -0.9, -0.3) based on the analysis using last observation carried forward (LOCF). With adjustments in antidiabetic background therapy after the initial 12 weeks, efficacy was maintained for 52 weeks, with an adjusted mean change from baseline in A1C of -0.7% compared to placebo (95% confidence interval -1.0, -0.4) based on analysis using LOCF.
16 HOW SUPPLIED/STORAGE AND HANDLING
JENTADUETO (linagliptin and metformin hydrochloride) tablets 2.5 mg/500 mg are supplied as follows:
Bottles of 60 (NDC 0597-0146-60)
Bottles of 180 (NDC 0597-0146-18)
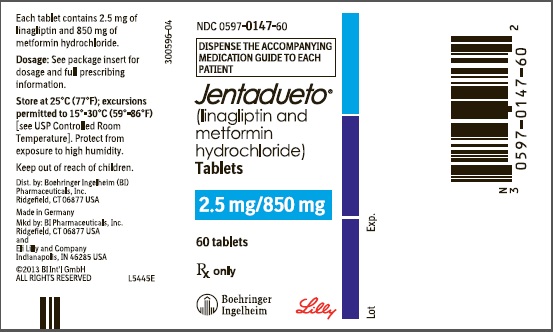
JENTADUETO (linagliptin and metformin hydrochloride) tablets 2.5 mg/850 mg are supplied as follows:
Bottles of 60 (NDC 0597-0147-60)
Bottles of 180 (NDC 0597-0147-18)
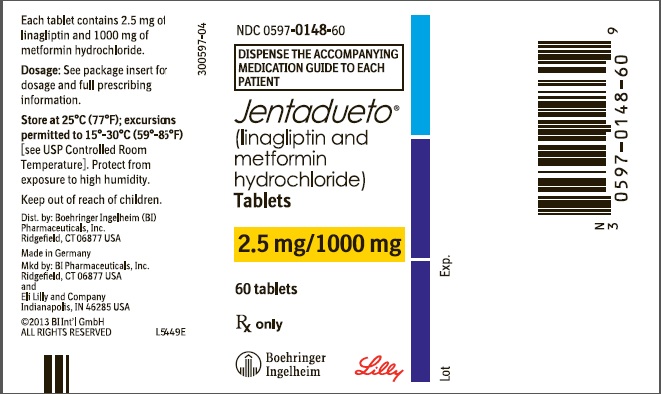
JENTADUETO (linagliptin and metformin hydrochloride) tablets 2.5 mg/1000 mg are supplied as follows:
Bottles of 60 (NDC 0597-0148-60)
Bottles of 180 (NDC 0597-0148-18)
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Protect from exposure to high humidity. Store in a safe place out of reach of children.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide)
17.1 Instructions
Inform patients of the potential risks and benefits of JENTADUETO and of alternative modes of therapy. Also inform patients about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and A1C testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. Advise patients to seek medical advice promptly during periods of stress such as fever, trauma, infection, or surgery, as medication requirements may change.
Inform patients of the risks of lactic acidosis due to the metformin component, its symptoms, and conditions that predispose to its development [see Warnings and Precautions (5.1)]. Advise patients to discontinue JENTADUETO immediately and to notify their doctor promptly if unexplained hyperventilation, malaise, myalgia, unusual somnolence, slow or irregular heart beat, sensation of feeling cold (especially in the extremities), or other nonspecific symptoms occur. GI symptoms are common during initiation of metformin treatment and may occur during initiation of JENTADUETO therapy; however, advise patients to consult their doctor if they develop unexplained symptoms. Although GI symptoms that occur after stabilization are unlikely to be drug related, such an occurrence of symptoms should be evaluated to determine if it may be due to metformin-induced lactic acidosis or other serious disease.
Inform patients that the risk of hypoglycemia is increased when JENTADUETO is used in combination with an insulin secretagogue (e.g., sulfonylurea), and that a lower dose of the insulin secretagogue may be required to reduce the risk of hypoglycemia.
Inform patients that acute pancreatitis has been reported during postmarketing use of linagliptin. Inform patients that persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to discontinue JENTADUETO promptly and contact their physician if persistent severe abdominal pain occurs [see Warnings and Precautions (5.2)].
Instruct patients to take JENTADUETO only as prescribed. If a dose is missed, advise patients not to double their next dose.
Warn patients against excessive alcohol intake, either acute or chronic, while receiving JENTADUETO [see Warnings and Precautions (5.6)].
Inform patients about the importance of regular testing of renal function and hematological parameters when receiving treatment with JENTADUETO.
Instruct patients to read the Medication Guide before starting JENTADUETO therapy and to reread each time the prescription is renewed. Instruct patients to inform their doctor if they develop any bothersome or unusual symptom, or if any symptom persists or worsens.
17.2 Laboratory Tests
Inform patients that response to all diabetic therapies should be monitored by periodic measurements of blood glucose and A1C levels, with a goal of decreasing these levels toward the normal range. A1C monitoring is especially useful for evaluating long-term glycemic control.
Initial and periodic monitoring of hematologic parameters (e.g., hemoglobin/hematocrit and red blood cell indices) and renal function (serum creatinine) should be performed, at least on an annual basis. While megaloblastic anemia has rarely been seen with metformin therapy, if this is suspected, Vitamin B12 deficiency should be excluded.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Marketed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
and
Eli Lilly and Company
Indianapolis, IN 46285 USA
Licensed from:
Boehringer Ingelheim International GmbH, Ingelheim, Germany
Copyright 2013 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
JENTADUETO (JEN ta doo e' toe)
(linagliptin and metformin hydrochloride)
Tablets
Read this Medication Guide carefully before you start taking JENTADUETO and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment. If you have any questions about JENTADUETO, ask your doctor or pharmacist.
What is the most important information I should know about JENTADUETO?
Serious side effects can happen in people taking JENTADUETO, including:
-
Lactic Acidosis. Metformin, one of the medicines in JENTADUETO, can cause a rare but serious condition called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in the hospital.
Stop taking JENTADUETO and call your doctor right away if you get any of the following symptoms of lactic acidosis:- feel very weak or tired
- have unusual (not normal) muscle pain
- have trouble breathing
- have unusual sleepiness or sleep longer than usual
- have sudden stomach or intestinal problems with nausea and vomiting or diarrhea
- feel cold, especially in your arms and legs
- feel dizzy or lightheaded
- have a slow or irregular heartbeat
- have kidney problems. People whose kidneys are not working properly should not take JENTADUETO.
- have liver problems
- have congestive heart failure that requires treatment with medicines
- drink alcohol very often, or drink a lot of alcohol in short-term ("binge" drinking)
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have certain x-ray tests with dyes or contrast agents that are injected into your body
- have surgery
- have a heart attack, severe infection, or stroke
- are 80 years of age or older and have not had your kidneys tested
-
Inflammation of the pancreas (pancreatitis) which may be severe and lead to death.
Certain medical problems make you more likely to get pancreatitis.
Before you start taking JENTADUETO:
Tell your doctor if you have ever had:
- inflammation of your pancreas (pancreatitis)
- stones in your gallbladder (gallstones)
- a history of alcoholism
- high blood triglyceride levels
Stop taking JENTADUETO and call your doctor right away if you have pain in your stomach area (abdomen) that is severe and will not go away. The pain may be felt going from your abdomen through to your back. The pain may happen with or without vomiting. These may be symptoms of pancreatitis.
- JENTADUETO is a prescription medicine that contains 2 diabetes medicines, linagliptin and metformin. JENTADUETO can be used along with diet and exercise to lower blood sugar in adults with type 2 diabetes when treatment with both linagliptin and metformin is appropriate.
- JENTADUETO is not for people with type 1 diabetes.
- JENTADUETO is not for people with diabetic ketoacidosis (increased ketones in the blood or urine).
- If you have had pancreatitis in the past, it is not known if you have a higher chance of getting pancreatitis while you take JENTADUETO.
- It is not known if JENTADUETO is safe and effective in children under 18 years of age.
Who should not take JENTADUETO?
-
Do not take JENTADUETO if you:
- have kidney problems
- have a condition called metabolic acidosis or diabetic ketoacidosis (increased ketones in the blood or urine).
- are allergic to linagliptin, metformin, or any of the ingredients in JENTADUETO. See the end of this Medication Guide for a complete list of ingredients in JENTADUETO.
- Symptoms of a serious allergic reaction to JENTADUETO may include:
- skin rash, itching, flaking or peeling
- raised red patches on your skin (hives)
- swelling of your face, lips, tongue and throat that may cause difficulty in breathing or swallowing
- difficulty with swallowing or breathing
What should I tell my doctor before using JENTADUETO?
-
Before you take JENTADUETO, tell your doctor if you:
- have or have had inflammation of your pancreas (pancreatitis).
- have kidney problems
- have liver problems
- have heart problems, including congestive heart failure
- drink alcohol very often, or drink a lot of alcohol in short term "binge" drinking
- are 80 years of age or older, you should not take JENTADUETO unless your kidneys have been checked and they are normal
- are going to get an injection of dye or contrast agents for an x-ray procedure. JENTADUETO will need to be stopped for a short time. Talk to your doctor about when you should stop JENTADUETO and when you should start JENTADUETO again. See "What is the most important information I should know about JENTADUETO?"
- have type 1 diabetes. JENTADUETO should not be used to treat people with type 1 diabetes.
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if JENTADUETO will harm your unborn baby. If you are pregnant, talk with your doctor about the best way to control your blood sugar while you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if JENTADUETO passes into your breast milk. Talk with your doctor about the best way to feed your baby if you take JENTADUETO.
-
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. JENTADUETO may affect the way other medicines work, and other medicines may affect how JENTADUETO works.
Especially tell your doctor if you take:- other medicines that can lower your blood sugar
- rifampin* (Rifadin®, Rimactane®, Rifater®, Rifamate®), an antibiotic that is used to treat tuberculosis
- Ask your doctor or pharmacist for a list of these medicines if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
- Take JENTADUETO exactly as your doctor tells you to take it.
- Take JENTADUETO 2 times each day with meals. Taking JENTADUETO with meals may lower your chance of having an upset stomach.
- If you miss a dose, take it with food as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take 2 doses of JENTADUETO at the same time.
- If you take too much JENTADUETO, call your doctor or Poison Control Center at 1-800-222-1222 or go to the nearest hospital emergency room right away.
- Your doctor may tell you to take JENTADUETO along with other diabetes medicines. Low blood sugar can happen more often when JENTADUETO is taken with certain other diabetes medicines. See "What are the possible side effects of JENTADUETO?"
- You may need to stop taking JENTADUETO for a short time. Call your doctor for instructions if you:
- are dehydrated (have lost too much body fluid). Dehydration can occur if you are sick with severe vomiting, diarrhea, or fever, or if you drink a lot less fluid than normal.
- plan to have surgery
- are going to get an injection of dye or contrast agent for an x-ray procedure. See "What is the most important information I should know about JENTADUETO?" and "Who should not take JENTADUETO?".
- When your body is under some types of stress, such as fever, trauma (such as a car accident), infection, or surgery, the amount of diabetes medicine that you need may change. Tell your doctor right away if you have any of these conditions and follow your doctor’s instructions.
- Check your blood sugar as your doctor tells you to.
- Stay on your prescribed diet and exercise program while taking JENTADUETO.
- Your doctor will check your diabetes with regular blood tests, including your blood sugar levels and your hemoglobin A1C.
- Your doctor will do blood tests to check how well your kidneys are working before and during your treatment with JENTADUETO.
What are the possible side effects of JENTADUETO tablets?
JENTADUETO may cause serious side effects, including:
- See "What is the most important information I should know about JENTADUETO?"
-
low blood sugar (hypoglycemia). If you take JENTADUETO with another medication that can cause low blood sugar, such as sulfonylurea or insulin, your risk of getting low blood sugar is higher. The dose of your sulfonylurea medicine or insulin may need to be lowered while you take JENTADUETO. Signs and symptoms of low blood sugar may include:
- headache
- hunger
- fast heart beat
- sweating
- feeling jittery
- irritability
- drowsiness
- weakness
- dizziness
- confusion
-
low blood sugar (hypoglycemia). If you take JENTADUETO with another medication that can cause low blood sugar, such as sulfonylurea or insulin, your risk of getting low blood sugar is higher. The dose of your sulfonylurea medicine or insulin may need to be lowered while you take JENTADUETO. Signs and symptoms of low blood sugar may include:
The most common side effects of JENTADUETO include:
- stuffy or runny nose and sore throat
- diarrhea
These are not all the possible side effects of JENTADUETO. For more information, ask your doctor or pharmacist.
Tell your doctor if you have any side effects that bother you or that do not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store JENTADUETO tablets?
- Store JENTADUETO between 68°F and 77°F (20°C and 25°C).
- Keep tablets dry.
Keep JENTADUETO and all medicines out of the reach of children.
General information about the safe and effective use of JENTADUETO
Medicines are sometimes prescribed for purposes other than those listed in Medication Guides. Do not use JENTADUETO for a condition for which it was not prescribed. Do not give JENTADUETO to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about JENTADUETO. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about JENTADUETO that is written for health professionals.
For more information, go to www.jentadueto.com or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257, or (TTY) 1-800-459-9906.
What are the ingredients in JENTADUETO?
Active Ingredients: linagliptin and metformin hydrochloride
Inactive Ingredients: arginine, corn starch, copovidone, colloidal silicon dioxide, magnesium stearate, titanium dioxide, propylene glycol, hypromellose, talc.
2.5 mg/500 mg and 2.5 mg/850 mg tablets also contain yellow ferric oxide.
2.5 mg/850 mg and 2.5 mg/1000 mg tablets also contain red ferric oxide.
Type 2 diabetes is a condition in which your body does not make enough insulin, and/or the insulin that your body produces does not work as well as it should. Your body can also make too much sugar. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems.
The main goal of treating diabetes is to lower your blood sugar to a normal level. High blood sugar can be lowered by diet and exercise, and by certain medicines when necessary.
Talk to your doctor about how to prevent, recognize, and take care of low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and other problems you have because of your diabetes.
This Medication Guide has been approved by the U. S. Food and Drug Administration.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Marketed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
and
Eli Lilly and Company
Indianapolis, IN 46285 USA
Licensed from:
Boehringer Ingelheim International GmbH
Ingelheim, Germany
*The brands listed are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc. The makers of these brands are not affiliated with and do not endorse Boehringer Ingelheim Pharmaceuticals, Inc., or its products.
Copyright 2013 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
| JENTADUETO
linagliptin tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| JENTADUETO
linagliptin tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| JENTADUETO
linagliptin tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | API MANUFACTURE(0597-0148, 0597-0146, 0597-0147), LABEL(0597-0148, 0597-0146, 0597-0147), PACK(0597-0147, 0597-0146, 0597-0148), MANUFACTURE(0597-0146, 0597-0147, 0597-0148), ANALYSIS(0597-0146, 0597-0147, 0597-0148) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Roxane Inc | 058839929 | LABEL(0597-0148, 0597-0146, 0597-0147), PACK(0597-0147, 0597-0146, 0597-0148) | |