KAOLIN PECTIN- kaolin and pectin, citrus suspension
Durvet, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
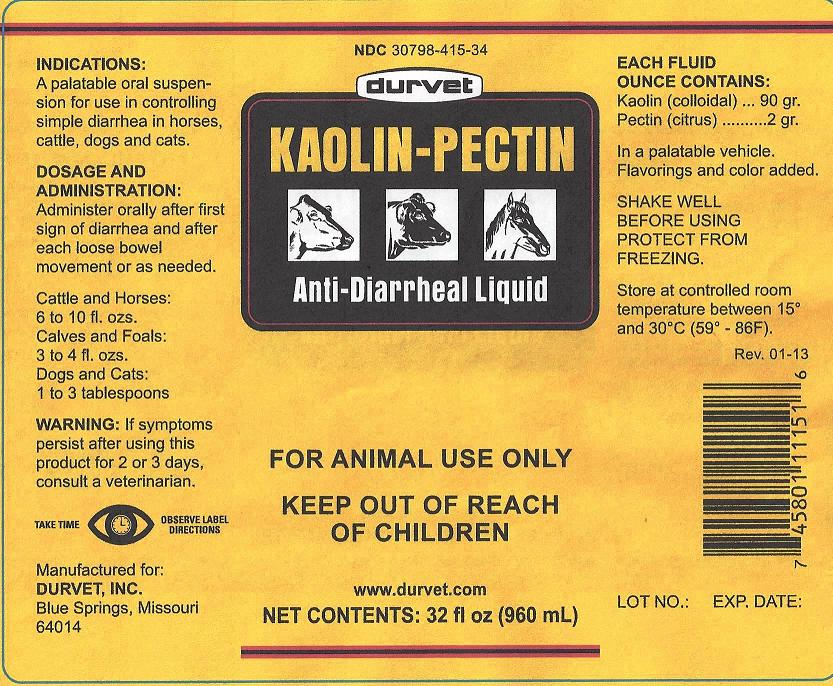
NDC 30798-415-34
durvet
KAOLIN-PECTIN
Anti-Diarrheal Liquid
FOR ANIMAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
NET CONTENTS: 32 fl oz (960ml)
INDICATIONS:
A palatable oral suspension for use in controlling simple diarrhea in horses, cattle, dogs and cats.
DOSAGE AND ADMINISTRATION:
Administer orally after first sign of diarrhea and after each loose bowel movement or as needed.
Cattle and Horses:
6 to 10 fl. ozs.
Calves and Foals:
3 to 4 fl. ozs.
Dogs and Cats:
1 to 3 tablespoons
WARNING:
If symptoms persist after using this product for 2 or 3 days, consult a veterinarian.
TAKE TIME OBSERVE LABEL DIRECTIONS
Manufactured for:
DURVET, INC.
Blue Springs, Missouri
64014
EACH FLUID OUNCE CONTAINS:
Kaolin (colloidal) ... 90 gr.
Pectin (citrus) ........... 2 gr.
In a palatable vehicle.
Flavorings and color added.
SHAKE WELL BEFORE USING
PROTECT FROM FREEZING.
| KAOLIN PECTIN
kaolin pectin suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Durvet, Inc. (056387798) |