OMEPRAZOLE
-
omeprazole capsule, delayed release pellets
KAISER FOUNDATION HOSPITALS
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONDOSAGE AND ADMINISTRATION
|
|
|
|
| Indication | Omeprazole Dose | Frequency |
| Short-Term Treatment of Active Duodenal Ulcer (2.1) | 20mg | Once daily for 4 weeks. Some patients may require an additional 4 weeks |
| H. Pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence (2.2) |
|
|
| Triple Therapy: |
|
|
| Omeprazole | 20mg | Each drug twice |
| Amoxicillin | 1000mg | daily for 10 days |
| Clarithromycin | 500mg |
|
|
|
|
|
| Dual Therapy: |
|
|
| Omeprazole | 40mg | Once daily for 14 days |
| Clarithromycin | 500mg | Three times daily for 14 days |
|
|
|
|
| Gastric Ulcer (2.3)
| 40mg | Once daily for 4 to 8 weeks |
| GERD (2.4)
| 20mg | Once daily for 4 to 8 weeks |
| Maintenance of Healing of Erosive Esophagitis (2.5)
| 20mg | Once daily |
| Pathological Hypersecretory Conditions (2.6)
| 60mg (varies with individual patient) | Once daily |
| Pediatric Patients
|
|
|
| (1 to 16 years of age) (2.7)
| Weight | Dose |
| GERD
| 5kg less than 10kg | 5mg Once Daily |
| And Maintenance of
| 10kg less than 20kg | 10mg |
| Healing of Erosive Esophagitis
| Equal or greater than 20kg | 20mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Patients should be informed that the omeprazole delayed-release capsules USP should be swallowed whole.
For patients unable to swallow an intact capsule, alternative administration options are available [See Dosage and Administration ( 2.8)].
2.1 Short-Term Treatment of Active Duodenal Ulcer
The recommended adult oral dose of omeprazole delayed-release capsules USP is 20 mg once daily. Most patients heal within four weeks. Some patients may require an additional four weeks of therapy.
2.3 Gastric Ulcer
The recommended adult oral dose is 40 mg once daily for 4 to 8 weeks.
2.4 Gastroesophageal Reflux Disease (GERD)
The recommended adult oral dose for the treatment of patients with symptomatic GERD and no esophageal lesions is 20 mg daily for up to 4 weeks. The recommended adult oral dose for the treatment of patients with erosive esophagitis and accompanying symptoms due to GERD is 20 mg daily for 4 to 8 weeks.
2.5 Maintenance of Healing of Erosive Esophagitis
The recommended adult oral dose is 20 mg daily. [See Clinical Studies ( 14.4)]
2.6 Pathological Hypersecretory Conditions
The dosage of omeprazole delayed-release capsules USP in patients with pathological hypersecretory conditions varies with the individual patient. The recommended adult oral starting dose is 60 mg once daily. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Doses up to 120 mg three times daily have been administered. Daily dosages of greater than 80 mg should be administered in divided doses. Some patients with Zollinger-Ellison syndrome have been treated continuously with omeprazole delayed-release capsules USP for more than 5 years.2.7 Pediatric Patients
For the treatment of GERD and maintenance of healing of erosive esophagitis, the recommended daily dose for pediatric patients- 1 to 16 years of age is as follows:
| Patient Weight | Omeprazole Daily Dose |
| 5kg less than 10kg | 5mg |
| 10kg less than 20kg | 10mg |
| Greater than 20kg | 20mg |
|
|
|
On a per kg basis, the doses of omeprazole required to heal erosive esophagitis in pediatric patients are greater than those for adults.
Alternative administrative options can be used for pediatric patients unable to swallow an intact capsule [See Dosage and Administration ( 2.8)].
2.8 Alternative Administration Options
Omeprazole is available as a delayed-release capsule USP.
For patients who have difficulty swallowing capsules, the contents of a omeprazole delayed-release capsules USP can be added to applesauce. One tablespoon of applesauce should be added to an empty bowl and the capsule should be opened. All of the pellets inside the capsule should be carefully emptied on the applesauce. The pellets should be mixed with the applesauce and then swallowed immediately with a glass of cool water to ensure complete swallowing of the pellets. The applesauce used should not be hot and should be soft enough to be swallowed without chewing. The pellets should not be chewed or crushed. The pellets/applesauce mixture should not be stored for future use.
Omeprazole delayed-release capsules USP, 10 mg, 20 mg and 40 mg ( 3)
Omeprazole delayed-release capsules USP, 10 mg, are opaque, hard gelatin, light green and white colored capsules, imprinted “Andrx 610” on the cap and “10 mg” on the body.Omeprazole delayed-release capsules USP, 20 mg, are opaque, hard gelatin, dark green and white colored capsules, imprinted “Andrx 620” on the cap and “20 mg” on the body.
Omeprazole delayed-release capsules, 40 mg, are opaque, hard gelatin, dark green and light green colored capsules, imprinted “Andrx 640” on the cap and “40 mg” on the body.
4 CONTRAINDICATIONS
Known hypersensitivity to any component of the formulation ( 4)
Omeprazole delayed-release capsules USP are contraindicated in patients with known hypersensitivity to any component of the formulation. Hypersensitivity reactions may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, interstitial nephritis, and urticaria [See Adverse Reactions ( 6)].
10 OVERDOSAGE
Reports have been received of overdosage with omeprazole in humans. Doses ranged up to 2400 mg (120 times the usual recommended clinical dose). Manifestations were variable, but included confusion, drowsiness, blurred vision, tachycardia, nausea, vomiting, diaphoresis, flushing, headache, dry mouth, and other adverse reactions similar to those seen in normal clinical experience. [See Adverse Reactions ( 6)] Symptoms were transient, and no serious clinical outcome has been reported when omeprazole delayed-release capsules USP was taken alone. No specific antidote for omeprazole overdosage is known. Omeprazole is extensively protein bound and is, therefore, not readily dialyzable. In the event of overdosage, treatment should be symptomatic and supportive.
As with the management of any overdose, the possibility of multiple drug ingestion should be considered. For current information on treatment of any drug overdose, contact a Poison Control Center at 1-800-222-1222.
Single oral doses of omeprazole at 1350, 1339, and 1200 mg/kg were lethal to mice, rats, and dogs, respectively. Animals given these doses showed sedation, ptosis, tremors, convulsions, and decreased activity, body temperature, and respiratory rate and increased depth of respiration.
11 DESCRIPTION
The active ingredient in Omeprazole Delayed-release Capsules USP is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C17H19N3O3S, with a molecular weight of 345.42.
Omeprazole is a white to off-white crystalline powder that melts with decomposition at about 155 degrees Celsius. It is a weak base, freely soluble in ethanol and methanol, and slightly soluble in acetone and isopropanol and very slightly soluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media, but has acceptable stability under alkaline conditions.
Omeprazole delayed-release capsules USP is supplied as delayed-release capsules for oral administration. Each delayed-release capsule contains either 10 mg, 20 mg or 40 mg of omeprazole in the form of enteric-coated granules with the following inactive ingredients: cetyl alcohol, disodium phosphate, hydroxy propyl methylcellulose phthalate, lactose anhydrous, povidone, sodium lauryl sulfate, sucrose and talc. The capsule shells and imprinting inks have the following inactive ingredients: ammonium hydroxide, D and C Yellow No.10, FD and C Blue No. 2 Aluminum Lake, FD and C Green No.3, gelatin-NF, propylene glycol, shellac and titanium dioxide.
15 REFERENCES
National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Fifth Edition. Approved Standard NCCLS Document M7-A5, Vol, 20, No. 2, NCCLS, Wayne, PA, January 2000.
16 HOW SUPPLIED/STORAGE AND HANDLING
Omeprazole Delayed-release Capsules USP, 40 mg, are opaque, hard gelatin, dark green and light green colored capsules, imprinted “Andrx 640” on cap and “40 mg” on the body. They are supplied as follows:
NDC 0179-0016-70 30 capsule in 1 Box, UNIT-DOSE
Store Omeprazole Delayed-release Capsules USP in a tight container protected from light and moisture. Store at controlled room temperature, 20° - 25°C (68° – 77°F). [See USP.]
17 PATIENT COUNSELING INFORMATION
Omeprazole delayed-release capsules USP should be taken before eating. Patients should be informed that the omeprazole delayed-release capsule USP should be swallowed whole.
For patients who have difficulty swallowing capsules, the contents of a omeprazole delayed-release capsule USP can be added to applesauce. One tablespoon of applesauce should be added to an empty bowl and the capsule should be opened. All of the pellets inside the capsule should be carefully emptied on the applesauce. The pellets should be mixed with the applesauce and then swallowed immediately with a glass of cool water to ensure complete swallowing of the pellets. The applesauce used should not be hot and should be soft enough to be swallowed without chewing. The pellets should not be chewed or crushed. The pellets/applesauce mixture should not be stored for future use.
Manufactured by:
Watson Laboratories, Inc.
Corona, CA 92880. USA
Distributed by:
Watson Pharma, Inc.
Rev. Date: 01/09
174697
Repackaged by:
Kaiser Foundation Hospitals
Livermore, CA 94551
Package Label - Principal Display Panel
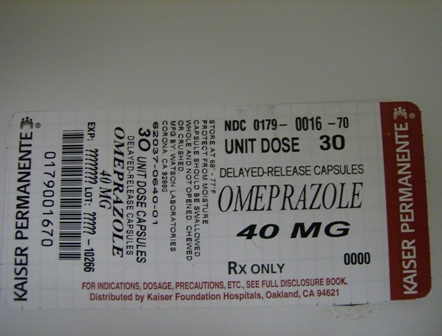
| OMEPRAZOLE
omeprazole capsule, delayed release pellets |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA075347 | 08/01/2009 | |
| Labeler - KAISER FOUNDATION HOSPITALS (053052619) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| KAISER FOUNDATION HOSPITALS | 053052619 | repack | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Watson Laboratories, Inc. | 840054118 | manufacture | |