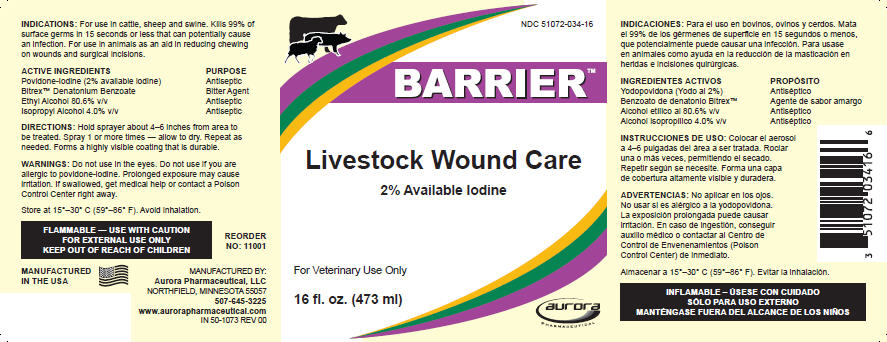
BARRIER LIVESTOCK WOUND CARE- povidone-iodine, alcohol, denatonium benzoate and isopropyl alcohol solution
Aurora Pharmaceutical LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Barrier®
Livestock Wound Care
INDICATIONS
For use in cattle, sheep and swine. Kills 99% of surface germs in 15 seconds or less that can potentially cause an infection. For use in animals as an aid in reducing chewing on wounds and surgical incisions.
| ACTIVE INGREDIENTS | PURPOSE |
|---|---|
| Povidone-Iodine (2% available iodine) | Antiseptic |
| Bitrex™ Denatonium Benzoate | Bitter Agent |
| Ethyl Alcohol 80.6% v/v | Antiseptic |
| Isopropyl Alcohol 4.0% v/v | Antiseptic |
DIRECTIONS
Hold sprayer about 4–6 inches from area to be treated. Spray 1 or more times — allow to dry. Repeat as needed. Forms a highly visible coating that is durable.
WARNINGS
Do not use in the eyes. Do not use if you are allergic to povidone-iodine. Prolonged exposure may cause irritation. If swallowed, get medical help or contact a Poison Control Center right away.
Store at 20°–25° C (68°–77° F). Excursions permitted between 15°–30° C (59°–86° F). Do not freeze. Avoid inhalation.
FLAMMABLE — USE WITH CAUTION
FOR EXTERNAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
WET HAIR OR FUR IS FLAMMABLE
Consumer Commodity ORM-D
| BARRIER
LIVESTOCK WOUND CARE
povidone-iodine, alcohol, denatonium benzoate, and isopropyl alcohol solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Aurora Pharmaceutical LLC (832848639) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurora Pharmaceutical LLC | 832848639 | MANUFACTURE | |