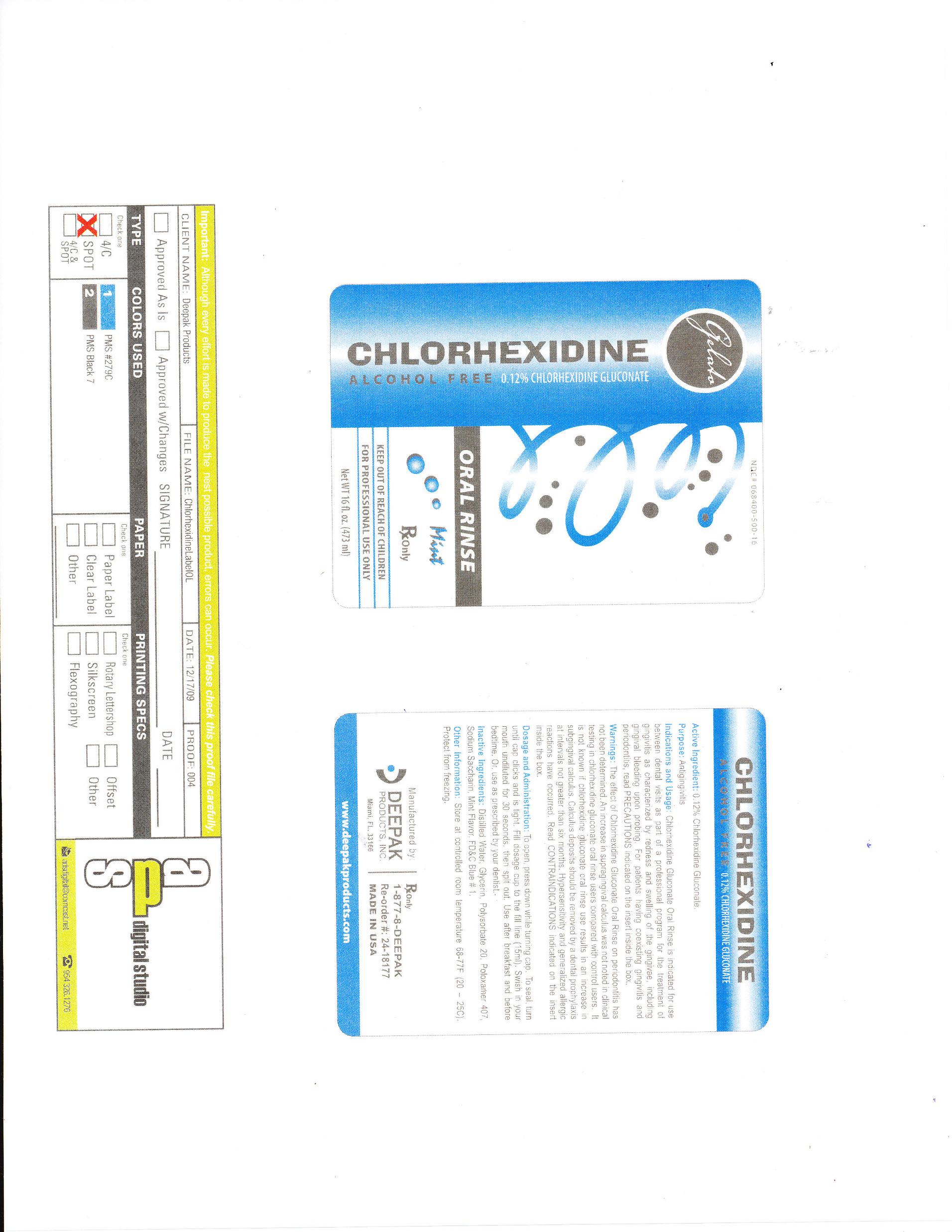
GELATO CHLOROHEXIDINE- chlorhexidine gluconate rinse
Mycone Dental Supply Co., Inc DBA Keystone Industries and Deepak Products Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Active Ingredient:
0.12% Chlorhexidine Gluconate
Indications and Usage:
Chlorhexidine Gluconate Oral Rinse is indictated for use between dental visits as part of a professional program for the treatment of gingivitis as characterized by redness and swelling of the gingivae, including gingival bleeding upon probing. For patients having coexisiting gingivitis and periodontitis, read PRECAUTIONS indicated on the insert inside the box.
Warnings:
The effect of Chlorhexidine Gluconate Oral Rinse on perodontitis has not been determined. An increase in supragingivitis was not noted in clinical testing in chlorhexidine gluconate oral rinse users compared with control users. It is not known if chlorhexidine gluconate oral rinse use results in an increase of subgingival calculus. Calculus deposits should be removed by dental prophylaxis at intervals not greater than six months. Hypersensitivity and generalized allergic reactions have occured. Read CONTRAINDICATIONS indicated on the insert inside the box.
Dosage and Administration:
To open, press down while turning cap. To seal, turn until cap clicks and is tight. Fill dosage cup to the fill line (15mL). Swish in your mouth undiluted for 30 seconds, then spit out. Use after breakfast and before bedtime. Or use as prescribed by your dentist.
Inactive Ingredients:
Distilled water, Glycerin, Polysorbate 20, Poloxamer 407, Sodium Saccharin, Mint Flavor, FD&C Blue #1.
Other Information:
Store at controlled room temperature 68-77°F (20-25°C). Protect from freezing.