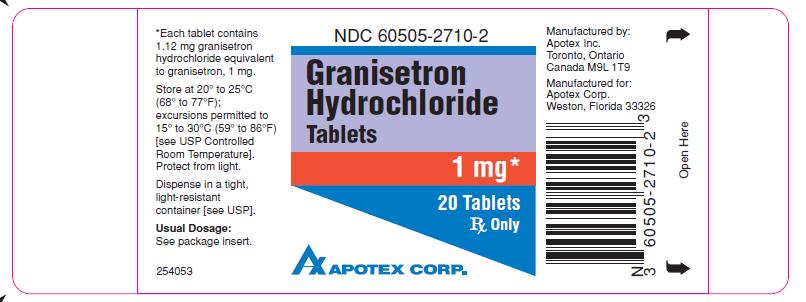
GRANISETRON HYDROCHLORIDE- granisetron hydrochloride tablet
Apotex Corp.
----------
DESCRIPTION
Granisetron Hydrochloride Tablets contain granisetron hydrochloride, an antinauseant andantiemetic agent. Chemically it is endo-N-(9-methyl-9-azabicyclo [3.3.1] non-3-yl)-1-methyl-1H-indazole-3-carboxamide hydrochloride with a molecular weight of 348.9 (312.4 freebase). Its empirical formula is C18H24N4O•HCl, while its chemical structure is:
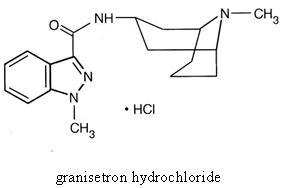
Granisetron hydrochloride is a white to off-white solid that is readily soluble in water andnormal saline at 20°C
Tablets for Oral Administration
Each white to off-white, round, biconvex, film-coated Granisetron Hydrochloride Tabletcontains 1.12 mg granisetron hydrochloride equivalent to granisetron, 1 mg. Inactiveingredients are: colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, lactosemonohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodiumstarch glycolate, and titanium dioxide.
CLINICAL PHARMACOLOGY
Granisetron is a selective 5-hydroxytryptamine3 (5-HT3) receptor antagonist with little or noaffinity for other serotonin receptors, including 5-HT1; 5-HT1A; 5-HT1B/C; 5-HT2; for alpha1-,alpha2-, or beta-adrenoreceptors; for dopamine-D2; or for histamine-H1; benzodiazepine;picrotoxin or opioid receptors.
Serotonin receptors of the 5-HT3 type are located peripherally on vagal nerve terminals andcentrally in the chemoreceptor trigger zone of the area postrema. During chemotherapy that induces vomiting, mucosal enterochromaffin cells release serotonin, which stimulates 5-HT3receptors. This evokes vagal afferent discharge, inducing vomiting. Animal studiesdemonstrate that, in binding to 5-HT3 receptors, granisetron blocks serotonin stimulation andsubsequent vomiting after emetogenic stimuli such as cisplatin. In the ferret animal model, asingle granisetron injection prevented vomiting due to high-dose cisplatin or arrestedvomiting within 5 to 30 seconds.
In most human studies, granisetron has had little effect on blood pressure, heart rate orECG. No evidence of an effect on plasma prolactin or aldosterone concentrations has beenfound in other studies.Following single and multiple oral doses, granisetron hydrochloride tablets slowed colonictransit in normal volunteers. However, granisetron hydrochloride had no effect on oro-cecaltransit time in normal volunteers when given as a single intravenous (IV) infusion of 50mcg/kg or 200 mcg/kg
Pharmacokinetics
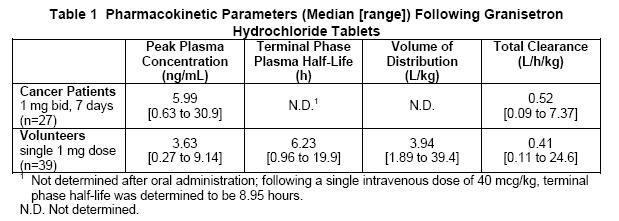
In healthy volunteers and adult cancer patients undergoing chemotherapy, administration of granisetron hydrochloride tablets produced mean pharmacokinetic data shown in Table 1.
Absorption
When granisetron hydrochloride tablets were administered with food, AUC was decreasedby 5% and Cmax increased by 30% in non-fasted healthy volunteers who received a singledose of 10 mg.
Distribution
Plasma protein binding is approximately 65% and granisetron distributes freely betweenplasma and red blood cells.
Metabolism
Granisetron metabolism involves N-demethylation and aromatic ring oxidation followed byconjugation. In vitro liver microsomal studies show that granisetron's major route ofmetabolism is inhibited by ketoconazole, suggestive of metabolism mediated by thecytochrome P-450 3A subfamily. Animal studies suggest that some of the metabolites mayalso have 5-HT3 receptor antagonist activity.
Elimination
Clearance is predominantly by hepatic metabolism. In normal volunteers, approximately11% of the orally administered dose is eliminated unchanged in the urine in 48 hours. Theremainder of the dose is excreted as metabolites, 48% in the urine and 38% in the feces.
Subpopulations
Gender
The effects of gender on the pharmacokinetics of granisetron hydrochloride tablets have notbeen studied. However, after intravenous infusion of granisetron hydrochloride, nodifference in mean AUC was found between males and females, although males had ahigher Cmax generally.In elderly and pediatric patients and in patients with renal failure or hepatic impairment, thepharmacokinetics of granisetron was determined following administration of intravenousgranisetron hydrochloride:
Elderly
The ranges of the pharmacokinetic parameters in elderly volunteers (mean age 71 years),given a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection, weregenerally similar to those in younger healthy volunteers; mean values were lower forclearance and longer for half-life in the elderly.
Renal Failure Patients
Total clearance of granisetron was not affected in patients with severe renal failure whoreceived a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection.Hepatically Impaired PatientsA pharmacokinetic study with intravenous granisetron hydrochloride in patients with hepaticimpairment due to neoplastic liver involvement showed that total clearance wasapproximately halved compared to patients without hepatic impairment. Given the widevariability in pharmacokinetic parameters noted in patients and the good tolerance of doseswell above the recommended dose, dosage adjustment in patients with possible hepaticfunctional impairment is not necessary.
Pediatric Patients
A pharmacokinetic study in pediatric cancer patients (2 to 16 years of age), given a single40 mcg/kg intravenous dose of granisetron hydrochloride injection, showed that volume ofdistribution and total clearance increased with age. No relationship with age was observedfor peak plasma concentration or terminal phase plasma half-life. When volume ofdistribution and total clearance are adjusted for body weight, the pharmacokinetics ofgranisetron are similar in pediatric and adult cancer patients.
CLINICAL TRIALS
Chemotherapy-Induced Nausea and Vomiting
Granisetron hydrochloride tablets prevent nausea and vomiting associated with initial andrepeat courses of emetogenic cancer therapy, as shown by 24-hour efficacy data fromstudies using both moderately- and highly-emetogenic chemotherapy.
Moderately Emetogenic Chemotherapy
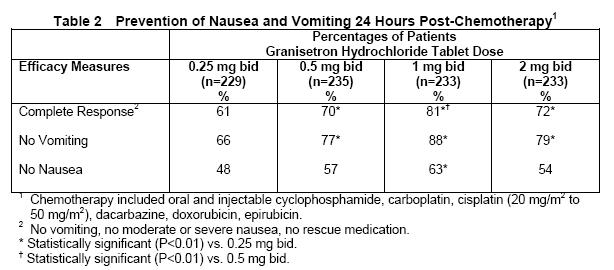
The first trial compared granisetron hydrochloride tablets doses of 0.25 mg to 2 mg bid, in930 cancer patients receiving, principally, cyclophosphamide, carboplatin, and cisplatin (20 mg/m2 to 50 mg/m2). Efficacy was based on complete response (ie, no vomiting, no moderate or severe nausea, no rescue medication), no vomiting, and no nausea. Table 2 summarizes the results of this study.
Results from a second double-blind, randomized trial evaluating granisetron hydrochloride tablets 2 mg qd and granisetron hydrochloride tablets 1 mg bid were compared to prochlorperazine 10 mg bid derived from a historical control. At 24 hours, there was no statistically significant difference in efficacy between the two granisetron hydrochloride tablet regimens. Both regimens were statistically superior to the prochlorperazine control regimen (see Table 3).
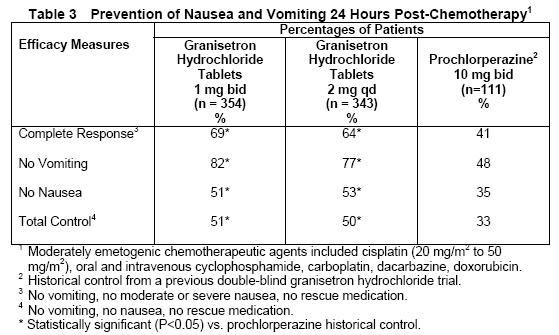
Results from a granisetron hydrochloride tablets 2 mg qd alone treatment arm in a third double-blind, randomized trial, were compared to prochlorperazine (PCPZ), 10 mg bid, derived from a historical control. The 24-hour results for granisetron hydrochloride tablets 2 mg qd were statistically superior to PCPZ for all efficacy parameters: complete response (58%), no vomiting (79%), no nausea (51%), total control (49%). The PCPZ rates are shown in Table 3.
The first double-blind trial compared granisetron hydrochloride tablets 1 mg bid, relative to placebo (historical control), in 119 cancer patients receiving high-dose cisplatin (mean dose 80 mg/m2). At 24 hours, granisetron hydrochloride tablets 1 mg bid was significantly (P<0.001) superior to placebo (historical control) in all efficacy parameters: complete response (52%), no vomiting (56%) and no nausea (45%). The placebo rates were 7%, 14%, and 7%, respectively, for the three efficacy parameters.
Results from a granisetron hydrochloride tablets 2 mg qd alone treatment arm in a second double-blind, randomized trial, were compared to both granisetron hydrochloride tablets 1 mg bid and placebo historical controls. The 24-hour results for granisetron hydrochloride tablets 2 mg qd were: complete response (44%), no vomiting (58%), no nausea (46%), total control (40%). The efficacy of granisetron hydrochloride tablets 2 mg qd was comparable to granisetron hydrochloride tablets 1 mg bid and statistically superior to placebo. The placebo rates were 7%, 14%, 7%, and 7%, respectively, for the four parameters.
No controlled study comparing granisetron injection with the oral formulation to prevent chemotherapy-induced nausea and vomiting has been performed.
Radiation-Induced Nausea and Vomiting
In a double-blind randomized study, 18 patients receiving granisetron hydrochloride tablets, 2 mg daily, experienced significantly greater antiemetic protection compared to patients in a historical negative control group who received conventional (non-5-HT3 antagonist) antiemetics. Total body irradiation consisted of 11 fractions of 120 cGy administered over 4 days, with three fractions on each of the first 3 days, and two fractions on the fourth day. Granisetron hydrochloride tablets were given one hour before the first radiation fraction of each day.
Twenty-two percent (22%) of patients treated with granisetron hydrochloride tablets did not experience vomiting or receive rescue antiemetics over the entire 4-day dosing period, compared to 0% of patients in the historical negative control group (P<0.01).
In addition, patients who received granisetron hydrochloride tablets also experienced significantly fewer emetic episodes during the first day of radiation and over the 4-day treatment period, compared to patients in the historical negative control group. The median time to the first emetic episode was 36 hours for patients who received granisetron hydrochloride tablets.
Fractionated Abdominal Radiation
The efficacy of granisetron hydrochloride tablets, 2 mg daily, was evaluated in a double-blind, placebo-controlled randomized trial of 260 patients. Granisetron hydrochloride tablets were given 1 hour before radiation, composed of up to 20 daily fractions of 180 to 300 cGy each. The exceptions were patients with seminoma or those receiving whole abdomen irradiation who initially received 150 cGy per fraction. Radiation was administered to the upper abdomen with a field size of at least 100 cm2.
The proportion of patients without emesis and those without nausea for granisetron hydrochloride tablets, compared to placebo, was statistically significant (P<0.0001) at 24 hours after radiation, irrespective of the radiation dose. Granisetron hydrochloride was superior to placebo in patients receiving up to 10 daily fractions of radiation, but was not superior to placebo in patients receiving 20 fractions.
Patients treated with granisetron hydrochloride tablets (n=134) had a significantly longer time to the first episode of vomiting (35 days vs. 9 days, P<0.001) relative to those patients who received placebo (n=126), and a significantly longer time to the first episode of nausea (11 days vs. 1 day, P<0.001). Granisetron hydrochloride provided significantly greater protection from nausea and vomiting than placebo.
INDICATIONS AND USAGE
Granisetron Hydrochloride Tablets are indicated for the prevention of:
- Nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin.
- Nausea and vomiting associated with radiation, including total body irradiation and fractionated abdominal radiation.
CONTRAINDICATIONS
Granisetron hydrochloride is contraindicated in patients with known hypersensitivity to the drug or any of its components.
PRECAUTIONS
Granisetron hydrochloride is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction. The use of Granisetron hydrochloride in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distention.
Drug Interactions
Granisetron does not induce or inhibit the cytochrome P-450 drug-metabolizing enzyme system in vitro. There have been no definitive drug-drug interaction studies to examine pharmacokinetic or pharmacodynamic interaction with other drugs; however, in humans, granisetron hydrochloride injection has been safely administered with drugs representing benzodiazepines, neuroleptics, and anti-ulcer medications commonly prescribed with antiemetic treatments. Granisetron hydrochloride injection also does not appear to interact with emetogenic cancer chemotherapies. Because granisetron is metabolized by hepatic cytochrome P-450 drug-metabolizing enzymes, inducers or inhibitors of these enzymes may change the clearance and, hence, the half-life of granisetron. No specific interaction studies have been conducted in anesthetized patients. In addition, the activity of the cytochrome P-450 subfamily 3A4 (involved in the metabolism of some of the main narcotic analgesic agents) is not modified by granisetron hydrochloride in vitro.
In in vitro human microsomal studies, ketoconazole inhibited ring oxidation of granisetron hydrochloride. However, the clinical significance of in vivo pharmacokinetic interactions with ketoconazole is not known. In a human pharmacokinetic study, hepatic enzyme induction with phenobarbital resulted in a 25% increase in total plasma clearance of intravenous granisetron hydrochloride. The clinical significance of this change is not known.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month carcinogenicity study, rats were treated orally with granisetron 1, 5 or 50 mg/kg/day (6, 30 or 300 mg/m2/day). The 50 mg/kg/day dose was reduced to 25 mg/kg/day (150 mg/m2/day) during week 59 due to toxicity. For a 50 kg person of average height (1.46 m2 body surface area), these doses represent 4, 20, and 101 times the recommended clinical dose (1.48 mg/m2, oral) on a body surface area basis. There was a statistically significant increase in the incidence of hepatocellular carcinomas and adenomas in males treated with 5 mg/kg/day (30 mg/m2/day, 20 times the recommended human dose based on body surface area) and above, and in females treated with 25 mg/kg/day (150 mg/m2/day, 101 times the recommended human dose based on body surface area). No increase in liver tumors was observed at a dose of 1 mg/kg/day (6 mg/m2/day, 4 times the recommended human dose based on body surface area) in males and 5 mg/kg/day (30 mg/m2/day, 20 times the recommended human dose based on body surface area) in females. In a 12-month oral toxicity study, treatment with granisetron 100 mg/kg/day (600 mg/m2/day, 405 times the recommended human dose based on body surface area) produced hepatocellular adenomas in male and female rats while no such tumors were found in the control rats. A 24-month mouse carcinogenicity study of granisetron did not show a statistically significant increase in tumor incidence, but the study was not conclusive.
Because of the tumor findings in rat studies, granisetron hydrochloride should be prescribed only at the dose and for the indication recommended (see INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION).
Granisetron was not mutagenic in in vitro Ames test and mouse lymphoma cell forward mutation assay, and in vivo mouse micronucleus test and in vitro and ex vivo rat hepatocyte UDS assays. It, however, produced a significant increase in UDS in HeLa cells in vitro and a significant increased incidence of cells with polyploidy in an in vitro human lymphocyte chromosomal aberration test.
Granisetron at oral doses up to 100 mg/kg/day (600 mg/m2/day, 405 times the recommended human dose based on body surface area) was found to have no effect on fertility and reproductive performance of male and female rats
Pregnancy: Teratogenic Effects: Pregnancy: Category B.
Reproduction studies have been performed in pregnant rats at oral doses up to 125 mg/kg/day (750 mg/m2/day, 507 times the recommended human dose based on body surface area) and pregnant rabbits at oral doses up to 32 mg/kg/day (378 mg/m2/day, 255 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to granisetron. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
ADVERSE REACTIONS
Chemotherapy-Induced Nausea and Vomiting
Over 3700 patients have received granisetron hydrochloride tablets in clinical trials with emetogenic cancer therapies consisting primarily of cyclophosphamide or cisplatin regimens.
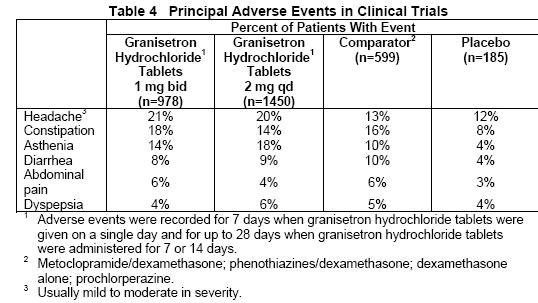
In patients receiving granisetron hydrochloride tablets 1 mg bid for 1, 7 or 14 days, or 2 mg qd for 1 day, adverse experiences reported in more than 5% of the patients with comparator and placebo incidences are listed in Table 4.
Other adverse events reported in clinical trials were:
Gastrointestinal: In single-day dosing studies in which adverse events were collected for 7 days, nausea (20%) and vomiting (12%) were recorded as adverse events after the 24-hour efficacy assessment period.
Hepatic: In comparative trials, elevation of AST and ALT (>2 times the upper limit of normal) following the administration of granisetron hydrochloride tablets occurred in 5% and 6% of patients, respectively. These frequencies were not significantly different from those seen with comparators (AST: 2%; ALT: 9%).
Cardiovascular: Hypertension (1%); hypotension, angina pectoris, atrial fibrillation, and syncope have been observed rarely.
Central Nervous System: Dizziness (5%), insomnia (5%), anxiety (2%), somnolence (1%). One case compatible with, but not diagnostic of, extrapyramidal symptoms has been reported in a patient treated with granisetron hydrochloride tablets.
Hypersensitivity: Rare cases of hypersensitivity reactions, sometimes severe (eg, anaphylaxis, shortness of breath, hypotension, urticaria) have been reported.
Other: Fever (5%). Events often associated with chemotherapy also have been reported: leukopenia (9%), decreased appetite (6%), anemia (4%), alopecia (3%), thrombocytopenia (2%).
Over 5000 patients have received injectable granisetron hydrochloride in clinical trials.
Table 5 gives the comparative frequencies of the five commonly reported adverse events (≥ 3%) in patients receiving granisetron hydrochloride injection, 40 mcg/kg, in single-day chemotherapy trials. These patients received chemotherapy, primarily cisplatin, and intravenous fluids during the 24-hour period following granisetron hydrochloride injection administration.
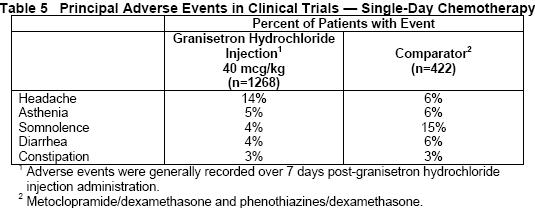
In the absence of a placebo group, there is uncertainty as to how many of these events should be attributed to granisetron hydrochloride, except for headache, which was clearly more frequent than in comparison groups.
Radiation-Induced Nausea and Vomiting
In controlled clinical trials, the adverse events reported by patients receiving granisetron hydrochloride tablets and concurrent radiation were similar to those reported by patients receiving granisetron hydrochloride tablets prior to chemotherapy. The most frequently reported adverse events were diarrhea, asthenia, and constipation. Headache, however, was less prevalent in this patient population.
OVERDOSAGE
There is no specific treatment for granisetron hydrochloride overdosage. In case of overdosage, symptomatic treatment should be given. Overdosage of up to 38.5 mg of granisetron hydrochloride injection has been reported without symptoms or only the occurrence of a slight headache.
DOSAGE AND ADMINISTRATION
Emetogenic Chemotherapy The recommended adult dosage of oral granisetron hydrochloride is 2 mg once daily or 1 mg twice daily. In the 2 mg once-daily regimen, two 1 mg tablets are given up to 1 hour before chemotherapy. In the 1 mg twice-daily regimen, the first 1 mg tablet is given up to 1 hour before chemotherapy, and the second tablet 12 hours after the first. Either regimen is administered only on the day(s) chemotherapy is given. Continued treatment, while not on chemotherapy, has not been found to be useful.
Use in the Elderly, Pediatric Patients, Renal Failure Patients or Hepatically Impaired Patients
No dosage adjustment is recommended (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
Radiation (Either Total Body Irradiation or Fractionated Abdominal Radiation)
The recommended adult dosage of oral granisetron hydrochloride is 2 mg once daily. Two 1 mg tablets are taken within 1 hour of radiation.
There is no experience with oral granisetron hydrochloride in the prevention of radiation-induced nausea and vomiting in pediatric patients.
No dosage adjustment is recommended.
HOW SUPPLIED
Granisetron Hydrochloride Tablets 1 mg are available as white to off-white, round, biconvex, film-coated tablets, engraved “APO” on one side, “GR” over “1” on the other side.
They are supplied as follows (intended for institutional use only):
Bottles of 20 (NDC 60505-2710-2)
Bottles of 1000 (NDC 60505-2710-8)
Unit Dose Blisters of 100: (NDC 60505-2710-0)
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container [see USP].
APOTEX INC.
GRANISETRON HYDROCHLORIDE TABLETS
1 mg
Manufactured by: Manufactured for:
Apotex Inc. Apotex Corp.
Toronto, Ontario Weston, Florida
Canada M9L 1T9 33326
Revised: March 2007
Rev. 0
| GRANISETRON HYDROCHLORIDE
granisetron hydrochloride tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Apotex Corp. (845263701) |
| Registrant - Apotex Inc (209429182) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Apotex Inc | 205576023 | manufacture(60505-2710), analysis(60505-2710) | |