CALCITONIN SALMON NASAL SPRAY
-
calcitonin, salmon spray, metered
Par Pharmaceutical Inc. (mgc)
----------
Calcitonin-SalmonNasal Spray, USP
Rx Only
Prescribing Information
DESCRIPTION
Calcitonin is a polypeptide hormone secreted by the parafollicular cells of the thyroid gland in mammals and by the ultimobranchial gland of birds and fish.
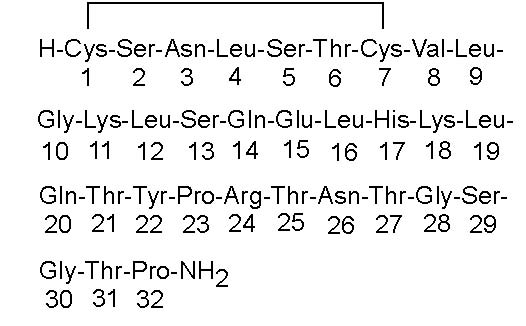
Calcitonin-Salmon Nasal Spray is a synthetic polypeptide of 32 amino acids in the same linear sequence that is found in calcitonin of salmon origin. This is shown by the following graphic formula:
It is provided in 3.8 mL fill glass bottles as a solution for nasal administration. This is sufficient medication for at least 30 doses.
Active Ingredient:calcitonin-salmon, 2200 I.U. per mL (corresponding to 200 I.U. per 0.09 mL actuation).
Inactive Ingredients:sodium chloride, chlorobutanol, hydrochloric acid (added as necessary to adjust pH), purified water and nitrogen.
The activity of Calcitonin-Salmon Nasal Spray is stated in International Units based on bioassay in comparison with the International Reference Preparation of calcitonin-salmon for Bioassay, distributed by the National Institute of Biologic Standards and Control, Holly Hill, London.
CLINICAL PHARMACOLOGY
Calcitonin acts primarily on bone, but direct renal effects and actions on the gastrointestinal tract are also recognized. Calcitonin-salmon appears to have actions essentially identical to calcitonins of mammalian origin, but its potency per mg is greater and it has a longer duration of action.
The information below, describing the clinical pharmacology of calcitonin, has been derived from studies with injectable calcitonin. The mean bioavailability of Calcitonin-Salmon Nasal Spray is approximately 3% of that of injectable calcitonin in normal subjects and, therefore, the conclusions concerning the CLINICAL PHARMACOLOGY of this preparation may be different.
The actions of calcitonin on bone and its role in normal human bone physiology are still not completely elucidated, although calcitonin receptors have been discovered in osteoclasts and osteoblasts.
Single injections of calcitonin cause a marked transient inhibition of the ongoing bone resorptive process. With prolonged use, there is a persistent, smaller decrease in the rate of bone resorption. Histologically, this is associated with a decreased number of osteoclasts and an apparent decrease in their resorptive activity. In vitro studies have shown that calcitonin-salmon causes inhibition of osteoclast function with loss of the ruffled osteoclast border responsible for resorption of bone. This activity resumes following removal of calcitonin-salmon from the test system. There is some evidence from the in vitro studies that bone formation may be augmented by calcitonin through increased osteoblastic activity.
Animal studies indicate that endogenous calcitonin, primarily through its action on bone, participates with parathyroid hormone in the homeostatic regulation of blood calcium. Thus, high blood calcium levels cause increased secretion of calcitonin which, in turn, inhibits bone resorption. This reduces the transfer of calcium from bone to blood and tends to return blood calcium towards the normal level. The importance of this process in humans has not been determined. In normal adults, who have a relatively low rate of bone resorption, the administration of exogenous calcitonin results in only a slight decrease in serum calcium in the limits of the normal range. In normal children and in patients with Paget’s disease in whom bone resorption is more rapid, decreases in serum calcium are more pronounced in response to calcitonin.
Bone biopsy and radial bone mass studies at baseline and after 26 months of daily injectable calcitonin indicate that calcitonin therapy results in formation of normal bone.
Postmenopausal Osteoporosis - Osteoporosis is a disease characterized by low bone mass and architectural deterioration of bone tissue leading to enhanced bone fragility and a consequent increase in fracture risk as patients approach or fall below a bone mineral density associated with increased frequency of fracture. The most common type of osteoporosis occurs in postmenopausal females. Osteoporosis is a result of a disproportionate rate of bone resorption compared to bone formation which disrupts the structural integrity of bone, rendering it more susceptible to fracture. The most common sites of these fractures are the vertebrae, hip, and distal forearm (Colles’ fractures). Vertebral fractures occur with the highest frequency and are associated with back pain, spinal deformity and a loss of height.
Calcitonin-Salmon Nasal Spray, given by the intranasal route, has been shown to increase spinal bone mass in post-menopausal women with established osteoporosis but not in early postmenopausal women.
Calcium Homeostasis - In two clinical studies designed to evaluate the pharmacodynamic response to Calcitonin-Salmon Nasal Spray, administration of 100-1600 I.U. to healthy volunteers resulted in rapid and sustained small decreases (but still within the normal range) in both total serum calcium and serum ionized calcium. Single doses greater than 400 I.U. did not produce any further biological response to the drug. The development of hypocalcemia has not been reported in studies in healthy volunteers or postmenopausal females.
Kidney - Studies with injectable calcitonin show increases in the excretion of filtered phosphate, calcium, and sodium by decreasing their tubular reabsorption. Comparable studies have not been carried out with Calcitonin-Salmon Nasal Spray.
Gastrointestinal Tract - Some evidence from studies with injectable preparations suggest that calcitonin may have significant actions on the gastrointestinal tract. Short-term administration of injectable calcitonin results in marked transient decreases in the volume and acidity of gastric juice and in the volume and the trypsin and amylase content of pancreatic juice. Whether these effects continue to be elicited after each injection of calcitonin during chronic therapy has not been investigated. These studies have not been conducted with Calcitonin-Salmon Nasal Spray.
Pharmacokinetics and Metabolism
The data on bioavailability of Calcitonin-Salmon Nasal Spray obtained by various investigators using different methods show great variability. Calcitonin-Salmon Nasal Spray is absorbed rapidly by the nasal mucosa. Peak plasma concentrations of drug appear 31-39 minutes after nasal administration compared to 16-25 minutes following parenteral dosing. In normal volunteers approximately 3% (range 0.3%-30.6%) of a nasally administered dose is bioavailable compared to the same dose administered by intramuscular injection. The half-life of elimination of calcitonin-salmon is calculated to be 43 minutes. There is no accumulation of the drug on repeated nasal administration at 10 hour intervals for up to 15 days. Absorption of nasally administered calcitonin has not been studied in postmenopausal women.
INDICATIONS AND USAGE
Postmenopausal Osteoporosis – Calcitonin-Salmon Nasal Spray is indicated for the treatment of postmenopausal osteoporosis in females greater than 5 years postmenopause with low bone mass relative to healthy premenopausal females. Calcitonin-Salmon Nasal Spray should be reserved for patients who refuse or cannot tolerate estrogens or in whom estrogens are contraindicated. Use of Calcitonin-Salmon Nasal Spray is recommended in conjunction with an adequate calcium (at least 1000 mg elemental calcium per day) and vitamin D (400 I.U. per day) intake to retard the progressive loss of bone mass. The evidence of efficacy is based on increases in spinal bone mineral density observed in clinical trials.
Two randomized, placebo controlled trials were conducted in 325 postmenopausal females [227 Calcitonin-Salmon Nasal Spray treated and 98 placebo treated] with spinal, forearm or femoral bone mineral density (BMD) at least one standard deviation below normal for healthy premenopausal females. These studies conducted over two years demonstrated that 200 I.U. daily of Calcitonin-Salmon Nasal Spray increases lumbar vertebral BMD relative to baseline and relative to placebo in osteoporotic females who were greater than 5 years postmenopause. Calcitonin-Salmon Nasal Spray produced statistically significant increases in lumbar vertebral BMD compared to placebo as early as six months after initiation of therapy with persistence of this level for up to 2 years of observation.
No effects of Calcitonin-Salmon Nasal Spray on cortical bone of the forearm or hip were demonstrated. However, in one study, BMD of the hip showed a statistically significant increase compared with placebo in a region composed of predominantly trabecular bone after one year of treatment changing to a trend at 2 years that was no longer statistically significant.
CONTRAINDICATIONS
Clinical allergy to calcitonin-salmon
WARNINGS
Allergic Reactions
Because calcitonin is a polypeptide, the possibility of a systemic allergic reaction exists. A few cases of allergic-type reactions have been reported in patients receiving Calcitonin-Salmon Nasal Spray. With injectable calcitonin-salmon there have been a few reports of serious allergic-type reactions (e.g., bronchospasm, swelling of the tongue or throat, anaphylactic shock, and in one case death attributed to anaphylaxis). The usual provisions should be made for the emergency treatment of such a reaction should it occur. Allergic reactions should be differentiated from generalized flushing and hypotension.
For patients with suspected sensitivity to calcitonin, skin testing should be considered prior to treatment utilizing a dilute, sterile solution of calcitonin-salmon injection. Physicians may wish to refer patients who require skin testing to an allergist. A detailed skin testing protocol is available from the Medical Services Department of MDRNA, Inc.
GENERAL PRECAUTIONS
Drug Interactions
Formal studies designed to evaluate drug interactions with calcitonin-salmon have not been done. No drug interaction studies have been performed with Calcitonin-Salmon Nasal Spray ingredients.
Currently, no drug interactions with calcitonin-salmon have been observed. The effects of prior use of diphosphonates in postmenopausal osteoporosis patients have not been assessed; however, in patients with Paget’s Disease prior diphosphonate use appears to reduce the anti-resorptive response to Calcitonin-Salmon Nasal Spray.
Periodic Nasal Examinations
Periodic nasal examinations with visualization of the nasal mucosa, turbinates, septum and mucosal blood vessel status are recommended.
The development of mucosal alterations or transient nasal conditions occurred in up to 9% of patients who received Calcitonin-Salmon Nasal Spray and in up to 12% of patients who received placebo nasal spray in studies in postmenopausal females. The majority of patients (approximately 90%) in whom nasal abnormalities were noted also reported nasally related complaints/symptoms as adverse events. Therefore, a nasal examination should be performed prior to start of treatment with nasal calcitonin and at any time nasal complaints occur.
In all postmenopausal patients treated with Calcitonin-Salmon Nasal Spray, the most commonly reported nasal adverse events included rhinitis (12%), epistaxis (3.5%), and sinusitis (2.3%). Smoking was shown not to have any contributory effect on the occurrence of nasal adverse events. One patient (0.3%) treated with Calcitonin-Salmon Nasal Spray who was receiving 400 I.U. daily developed a small nasal wound. In clinical trials in another disorder (Paget’s Disease), 2.8% of patients developed nasal ulcerations.
If severe ulceration of the nasal mucosa occurs, as indicated by ulcers greater than 1.5 mm in diameter or penetrating below the mucosa, or those associated with heavy bleeding, Calcitonin-Salmon Nasal Spray should be discontinued. Although smaller ulcers often heal without withdrawal of Calcitonin-Salmon Nasal Spray, medication should be discontinued temporarily until healing occurs.
Information for Patients
Careful instructions on pump assembly, priming of the pump and nasal introduction of Calcitonin-Salmon Nasal Spray should be given to the patient. Although instructions for patients are supplied with individual bottles, procedures for use should be demonstrated to each patient. Patients should notify their physician if they develop significant nasal irritation.
Patients should be advised of the following:
- Store new, unassembled bottles in the refrigerator between 2°C-8°C (36°F-46°F).
- Protect the product from freezing.
- Before priming the pump and using a new bottle, allow it to reach room temperature.
- Store bottle in use at room temperature 15°C-30°C (59°F-86°F) in an upright position, for up to 35 days. Each bottle contains at least 30 doses.
- See DOSAGE AND ADMINISTRATION, Priming (Activation) of Pump for complete instructions on priming the pump and administering Calcitonin-Salmon Nasal Spray.
You should keep track of the number of doses used from the bottle.
After 30 doses, each spray may not deliver the correct amount of medication, even if the bottle is not completely empty.
Laboratory Tests
Urine sediment abnormalities have not been reported in ambulatory volunteers treated with Calcitonin-Salmon Nasal Spray. Coarse granular casts containing renal tubular epithelial cells were reported in young adult volunteers at bed rest who were given injectable calcitonin-salmon to study the effect of immobilization on osteoporosis. There was no evidence of renal abnormality and the urine sediment became normal after calcitonin was stopped. Periodic examinations of urine sediment should be considered.
Carcinogenesis and Mutagenesis and Impairment of Fertility
An increased incidence of non-functioning pituitary adenomas has been observed in one-year toxicity studies in Sprague-Dawley and Fischer 344 Rats administered (subcutaneously) calcitonin-salmon at dosages of 80 I.U. per kilogram per day (16-19 times the recommended human parenteral dose and about 130-160 times the human intranasal dose based on body surface area). The findings suggest that calcitonin-salmon reduced the latency period for development of pituitary adenomas that do not produce hormones, probably through the perturbation of physiologic processes involved in the evolution of this commonly occurring endocrine lesion in the rat. Although administration of calcitonin- salmon reduces the latency period of the development of nonfunctional proliferative lesions in rats, it did not induce the hyperplastic/neoplastic process.
Calcitonin-salmon was tested for mutagenicity using Salmonella typhimurium (5 strains) and Escherichia coli (2 strains), with and without rat liver metabolic activation, and found to be non-mutagenic. The drug was also not mutagenic in a chromosome aberration test in mammalian V79 cells of the Chinese Hamster in vitro.
Pregnancy
Teratogenic Effects
Category C
Calcitonin-salmon has been shown to cause a decrease in fetal birth weights in rabbits when given by injection in doses 8-33 times the parenteral dose and 70-278 times the intranasal dose recommended for human use based on body surface area.
Since calcitonin does not cross the placental barrier, this finding may be due to metabolic effects on the pregnant animal. There are no adequate and well controlled studies in pregnant women with calcitonin-salmon. Calcitonin-Salmon Nasal Spray is not indicated for use in pregnancy.
Nursing Mothers
It is not known whether this drug is excreted in human milk. As a general rule, nursing should not be undertaken while a patient is on this drug since many drugs are excreted in human milk. Calcitonin has been shown to inhibit lactation in animals.
Pediatric Use
There are no data to support the use of Calcitonin-Salmon Nasal Spray in children. Disorders of bone in children referred to as idiopathic juvenile osteoporosis have been reported rarely. The relationship of these disorders to postmenopausal osteoporosis has not been established and experience with the use of calcitonin in these disorders is very limited.
Geriatric Use
In one large multi-centered, double-blind, randomized clinical study of Calcitonin-Salmon Nasal Spray, 279 patients were less than 65 years old, while 467 patients were 65 to 74 years old and 196 patients were 75 and over. Compared to subjects less than 65 years old, the incidence of nasal adverse events (rhinitis, irritation, erythema, and excoriation) was higher in patients over the age of 65, particularly those over the age of 75. Most events were mild in intensity. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
ADVERSE REACTIONS
The incidence of adverse reactions reported in studies involving postmenopausal osteoporotic patients chronically exposed to Calcitonin-Salmon Nasal Spray (N=341) and to placebo nasal spray (N=131) and reported in greater than 3% of Calcitonin-Salmon Nasal Spray treated patients are presented below in the following table. Most adverse reactions were mild to moderate in severity. Nasal adverse events were most common with 70% mild, 25% moderate, and 5% severe in nature (placebo rates were 71% mild, 27% moderate, and 2% severe).
In addition, the following adverse events were reported in fewer than 3% of patients during chronic therapy with Calcitonin-Salmon Nasal Spray. Adverse events reported in 1%-3% of patients are identified with an asterisk(*). The remainder occurred in less than 1% of patients. Other than flushing, nausea, possible allergic reactions, and possible local irritative effects in the respiratory tract, a relationship to Calcitonin-Salmon Nasal Spray has not been established.
Body as a whole – General Disorders: influenza-like symptoms*, fatigue*, periorbital edema, fever
Integumentary:erythematous rash*, skin ulceration, eczema, alopecia, pruritus, increased sweating
Musculoskeletal/Collagen:arthrosis*, myalgia*, arthritis, polymyalgia rheumatica, stiffness
Respiratory/Special Senses:sinusitis*, upper respiratory tract infection*, bronchospasm*, pharyngitis, bronchitis, pneumonia, coughing, dyspnea, taste perversion, parosmia
Cardiovascular:hypertension*, angina pectoris*, tachycardia, palpitation, bundle branch block, myocardial infarction
Gastrointestinal:dyspepsia*, constipation*, abdominal pain*, nausea*, diarrhea*, vomiting, flatulence, increased appetite, gastritis, dry mouth
Liver/Metabolic:cholelithiasis, hepatitis, thirst, weight increase
Endocrine:goiter, hyperthyroidism
Urinary System:cystitis*, pyelonephritis, hematuria, renal calculus
Central and Peripheral Nervous System:dizziness*, paresthesia*, vertigo, migraine, neuralgia, agitation
Hearing/Vestibular:tinnitus, hearing loss, earache
Vision:abnormal lacrimation*, conjunctivitis*, blurred vision, vitreous floater
Vascular:flushing, cerebrovascular accident, thrombophlebitis
Hematologic/Resistance Mechanisms:lymphadenopathy*, infection*, anemia
Psychiatric:depression*, insomnia, anxiety, anorexia
Common adverse reactions associated with the use of injectable calcitonin-salmon occurred less frequently in patients treated with Calcitonin-Salmon Nasal Spray than in those patients treated with injectable calcitonin. Nausea, with or without vomiting, which occurred in 1.8% of patients treated with the nasal spray (and 1.5% of those receiving placebo nasal spray) occurs in about 10% of patients who take injectable calcitonin-salmon. Flushing, which occurred in less than 1% of patients treated with the Nasal Spray, occurs in 2%-5% of patients treated with injectable calcitonin-salmon. Although the administered dosages of injectable and nasal spray calcitonin-salmon are comparable (50-100 units daily of injectable versus 200 units daily of nasal spray), the nasal dosage form has a mean bioavailability of about 3% (range 0.3%-30.6%) and therefore provides less drug to the systemic circulation, possibly accounting for the decrease in frequency of adverse reactions.
The collective foreign marketing experience with Calcitonin-Salmon Nasal Spray does not show evidence of any notable difference in the incidence profile of reported adverse reactions when compared with that seen in the clinical trials.
OVERDOSAGE
No instances of overdose with Calcitonin-Salmon Nasal Spray have been reported and no serious adverse reactions have been associated with high doses. There is no known potential for drug abuse for calcitonin-salmon.
Single doses of Calcitonin-Salmon Nasal Spray up to 1600 I.U., doses up to 800 I.U. per day for three days and chronic administration of doses up to 600 I.U. per day have been studied without serious adverse effects. A dose of 1000 I.U. of Calcitonin-Salmon injectable solution given subcutaneously may produce nausea and vomiting. A dose of Calcitonin-Salmon injectable solution of 32 I.U. per kg per day for one or two days demonstrated no additional adverse effects.
There have been no reports of hypocalcemic tetany. However, the pharmacologic actions of Calcitonin-Salmon Nasal Spray suggest that this could occur in overdose. Therefore, provisions for parenteral administration of calcium should be available for the treatment of overdose.
DOSAGE AND ADMINISTRATION
The recommended dose of Calcitonin-Salmon Nasal Spray in postmenopausal osteoporotic females is one spray (200 I.U.) per day administered intranasally, alternating nostrils daily.
Drug effect may be monitored by periodic measurements of lumbar vertebral bone mass to document stabilization of bone loss or increases in bone density. Effects of Calcitonin-Salmon Nasal Spray on biochemical markers of bone turnover have not been consistently demonstrated in studies in postmenopausal osteoporosis. Therefore, these parameters should not be solely utilized to determine clinical response to Calcitonin-Salmon Nasal Spray therapy in these patients.
Priming (Activation) of Pump
Before the first dose and administration, Calcitonin-Salmon Nasal Spray should be at room temperature. To prime the pump, the bottle should be held upright and the two white side arms of the pump depressed toward the bottle until a full spray is produced. The pump is primed once the first full spray is emitted. To administer, the nozzle should be carefully placed into the nostril with the head in the upright position, and the pump firmly depressed toward the bottle. The pump should not be primed before each daily dose.
HOW SUPPLIED
Calcitonin-Salmon Nasal Spray, USP
Available as a metered dose clear solution in a 3.8 mL fill clear glass bottle. It is available in a dosage strength of 200 I.U. per activation (0.09 mL/spray). A screw-on pump is provided. The pump, following priming, will deliver 0.09 mL of solution. Calcitonin-Salmon Nasal Spray contains 2200 I.U./mL calcitonin-salmon and is provided in an individual box containing one glass bottle and one screw-on pump (NDC 49884-161-11).
Store and Dispense
Store unopened bottle in refrigerator between 2°C-8°C (36°F-46°F). Protect from freezing.
Store bottle in use at room temperature between 20°C-25°C (68°F-77°F) in an upright position, for up to 35 days. Each bottle contains at least 30 doses.
Distributed by: Par Pharmaceutical Companies, Inc.
PRINCIPAL DISPLAY PANEL – CONTAINER LABEL
PRINCIPAL DISPLAY PANEL - CARTON
| CALCITONIN SALMON
NASAL SPRAY
calcitonin salmon spray, metered |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA076979 | 06/08/2009 | |
| Labeler - Par Pharmaceutical Inc. (mgc) (092733690) |
| Registrant - Par Pharmaceutical Inc (092733690) |