DEXTROSE- dextrose monohydrate injection, solution
Hospira, Inc.
----------
Hospira
Plastic THERMOJECT®
Unit of Use System
For Thermodilution Measurement
of Cardiac Output
Rx Only
DESCRIPTION
5% Dextrose Injection, USP is a sterile, nonpyrogenic solution of dextrose in water for injection. It is administered via a thermodilution catheter for measuring cardiac output. Each mL contains dextrose, hydrous 50 mg. Osmolar concentration is 0.253 mOsmol/mL (calc). pH is 5.6 (3.5 to 6.5). The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended for use only as a single-dose thermodilution agent. When smaller amounts are required the unused portion should be discarded.
Used as directed, the Thermoject Unit of Use System provides for aseptic delivery of cardiac output injectate. The solution may be used iced or at room temperature for injection of measured boluses from 1 to 10 mL as required.
5% Dextrose Injection, USP in the Thermoject System for cardiac output monitoring may be classified as a thermodilution diagnostic aid.
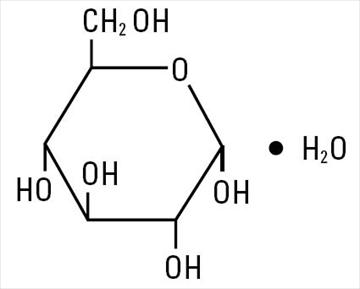
Dextrose, USP is chemically designated D-glucose, monohydrate (C2H12O6 • H2O), a hexose sugar freely soluble in water. It has the following structural formula:
The semi-rigid vial is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
CLINICAL PHARMACOLOGY
Used as a thermodilution agent for measuring cardiac output, 5% Dextrose Injection, USP usually involves volumes too small to affect carbohydrate or water balance except possibly in newborn or very small infants. Dextrose injected parenterally undergoes eventual absorption and oxidation to carbon dioxide and water.
INDICATIONS AND USAGE
5% Dextrose Injection, USP in the Thermoject System is indicated for measurement of cardiac output by the thermodilution method. Use only for Thermodilution as directed.
CONTRAINDICATIONS
This solution is not intended and should not be used for injection by the usual parenteral routes of administration, including infusion via intravenous administration sets.
PRECAUTIONS
Do not use unless the solution is clear and container undamaged. Discard unused portion. Use only for Thermodilution as directed. The solution should be inspected visually for particulate matter and discoloration prior to use.
Pregnancy Category C. Animal reproduction studies have not been conducted with dextrose. It is also not known whether dextrose can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dextrose should be given to a pregnant woman only if clearly needed.
Pediatric Use: The safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. In neonates or very small infants the volume of fluid may affect fluid and electrolyte balance.
Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, tissue necrosis or infection, local or systemic.
If an adverse reaction does occur, discontinue the injection, evaluate the patient and institute appropriate countermeasures.
OVERDOSAGE
In a small volume container, the solution is unlikely to pose a threat of solute or fluid overload except possibly in newborn or very small infants.
DOSAGE AND ADMINISTRATION
The volume of bolus injection (1 to 10 mL) varies depending on patient condition and requirements to produce an adequate thermodilution cardiac output curve. Usually the smallest volume necessary to produce an adequate curve is used so as to limit fluid dosage.
As reported in the literature, the dosage and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia.
When cardiac output measurement is desired, the vial and injector are connected as shown under HOW SUPPLIED. Prime the injector. The injector is connected securely to the side port of a 3-way stopcock attached to the RA proximal port of a thermodilution catheter using the male luer lock. The hand should be used to cradle the stopcock and Thermoject Syringe so a smooth, quick injection can be made.
CAUTION: Handle vial as little as possible to prevent change in solution temperature.
TO ADD SUBSEQUENT VIALS: Properly position the 3-way stopcock and disconnect the unit. Remove the empty vial by rotating counterclockwise. Attach replacement vial as before, i.e., rotate vial clockwise three times. Prime the injector and reconnect the unit to the stopcock.
The final vial used in a series of injections should be left in the injector so the system remains closed in case subsequent cardiac output measurements are made.
HOW SUPPLIED
|
Contents |
Product |
|
|
Syringe Kit List No. 1082 Qty. |
Syringe w/Luer Lock List No. 1080 Qty. |
|
|
10 mL Thermoject Vials, |
4 |
1 |
|
10 mL Thermoject |
1 |
1 |
|
Empty Vial (For |
1 |
0 |
|
Carrying Tray |
1 |
0 |
USE ASEPTIC TECHNIQUE
- Open carton using tear strip, remove carrying tray and contents.
- Remove injector from tray; if iced injectate is desired, submerge tray into ice bath without submerging the yellow vial caps.
- Remove purple cap from empty vial, fill vial with desired solution, replace cap and place injectate probe through hole in cap into solution (tip should be submerged at least 3 cm).
DISPENSING
CAUTION: Do not connect injector and vial until ready to use.
1. When desired solution temperature is reached, remove cap from injector and one vial.
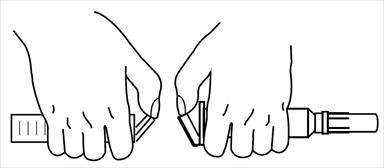
2. Insert vial into injector and rotate clockwise (about three turns) until medication enters needle.
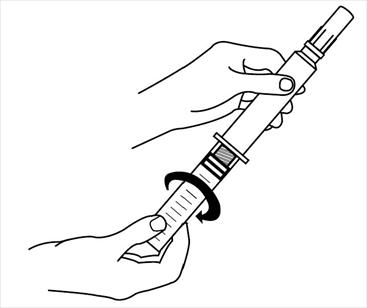
CAUTION: Handle vial as little as possible to prevent change in solution temperature.
3. Twist and pull luer cover to remove.
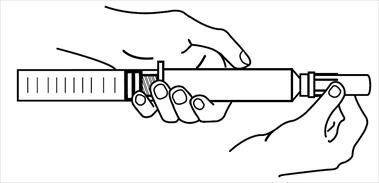
4. Hold vial and injector in vertical position with vial below injector and prime the injector to remove all air from vial and injector. Connect injector securely to the side port of a 3-way stopcock attached to the RA proximal port of a thermodilution catheter.
5. TO ADD SUBSEQUENT VIALS. Properly position the 3-way stopcock and disconnect the unit. Remove the empty vial by rotating counterclockwise. Attach replacement vial and rotate vial clockwise three times. Prime the injector and reconnect the unit to the stopcock.
6. Leave the last vial in the series in the injector to prevent contamination of the needle and fluid path.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Revised: June, 2005
Hospira
Hospira, Inc.
Lake Forest, IL 60045 USA
©Hospira 2005 EN-0956 Printed in USA
| DEXTROSE
dextrose monohydrate injection, solution |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| DEXTROSE
dextrose monohydrate injection, solution |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Hospira, Inc. (141588017) |

