COMPLETE-RF PRENATAL- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, iron, magnesium oxide, zinc oxide, cupric oxide and docusate sodium tablet, film coated
TRIGEN Laboratories, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION
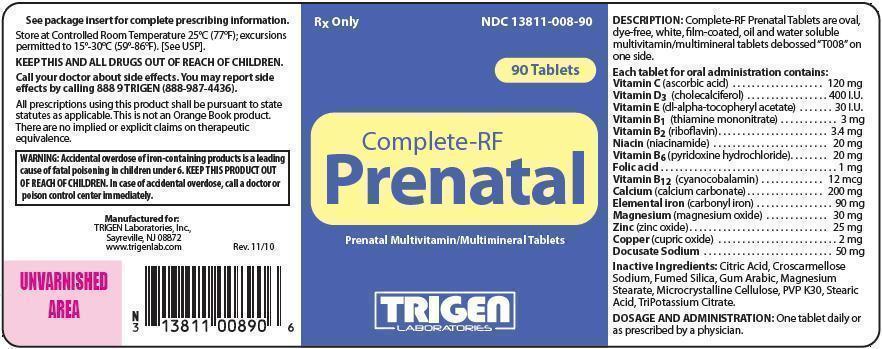
Complete-RF Prenatal Tablets are oval, dye free, white, film-coated, oil and water soluble multivitamin/multimineral tablets debossed "T008" on one side.
| Each tablet for oral administration contains: | |
| Vitamin C (ascorbic acid) | 120 mg |
| Vitamin D3 (cholecalciferol) | 400 IU |
| Vitamin E (dl-alpha tocopheryl acetate) | 30 IU |
| Vitamin B1 (thiamine mononitrate) | 3 mg |
| Vitamin B2 (riboflavin) | 3.4 mg |
| Niacin (niacinamide) | 20 mg |
| Vitamin B6 (pyridoxine hydrochloride) | 20 mg |
| Folic Acid | 1 mg |
| Vitamin B12 (cyanocobalamin) | 12 mcg |
| Calcium (calcium carbonate) | 200 mg |
| Elemental Iron (carbonyl iron) | 90 mg |
| Magnesium (magnesium oxide) | 30 mg |
| Zinc (zinc oxide) | 25 mg |
| Copper (cupric oxide) | 2 mg |
| Docusate Sodium | 50 mg |
INDICATIONS AND USAGE
Complete-RF Prenatal is a multivitamin/multimineral nutritional supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers. Complete-RF Prenatal can also be beneficial in improving the nutritional status of women prior to conception.
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
WARNINGS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
PRECAUTIONS
Geriatric Use
Clinical studies on this product have not been performed in sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac functions, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Adverse reactions with iron therapy may include constipation, diarrhea, nausea, vomiting, dark stools and abdominal pain. Adverse reactions with iron therapy are usually transient. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Rx Only
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. There are no implied or explicit claims on therapeutic equivalence.
Manufactured for:
TRIGEN Laboratories, Inc.
Sayreville, NJ 08872
www.trigenlab.com
MADE IN CANADA
Rev. 11/10
| COMPLETE-RF PRENATAL
ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, iron, magnesium oxide, zinc oxide, cupric oxide, and docusate sodium tablet, film coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - TRIGEN Laboratories, Inc. (830479668) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Viva Pharmaceutical, Inc. | 963714766 | manufacture(13811-008) | |