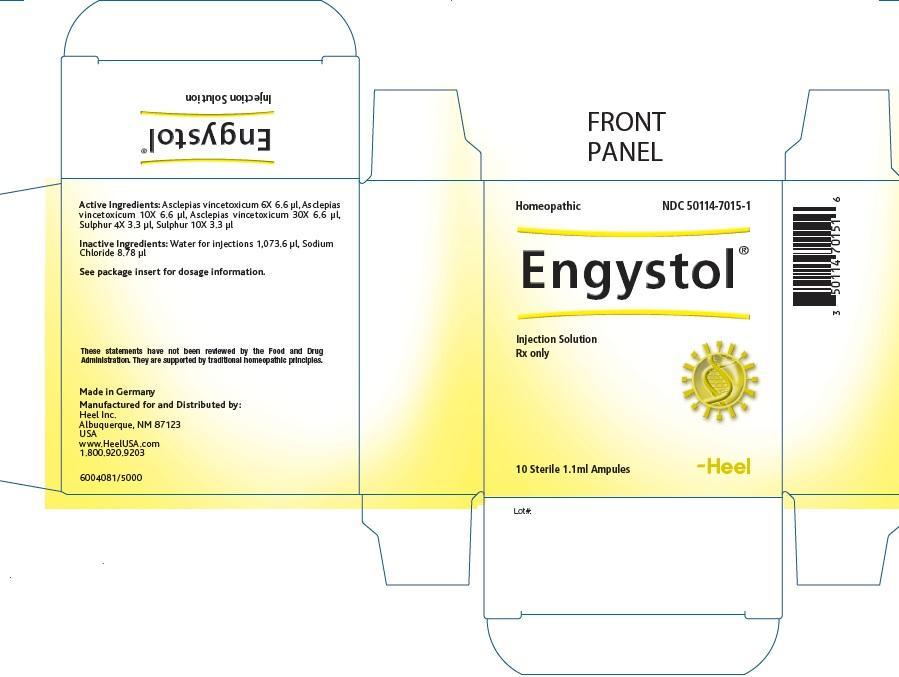
ENGYSTOL - cynanchum vincetoxicum root and sulfur injection
Heel Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION
| Each 1.1 ml solution for injection ampule contains: | |||
| Active Ingredients: | |||
| Ingredient name | Potency | Quantity | Final dilution |
| Asclepias vincetoxicum | 6X | 6.6 µl | 8.22X |
| Asclepias vincetoxicum | 10X | 6.6 µl | 12.22X |
| Asclepias vincetoxicum | 30X | 6.6 µl | 32.22X |
| Sulphur | 4X | 3.3 µl | 6.52X |
| Sulphur | 10X | 3.3 µl | 12.52X |
Inactive Ingredients:
Water for injection 1,073.6 μl
Sodium Chloride 8.78 μl
INDICATIONS AND USAGE
Engystol® Injection Solution is a homeopathic drug product indicated for the support of the immune system to reduce severity and duration of symptoms in viral infections, particularly in the early stages of colds and influenza-like illnesses.
DOSAGE AND ADMINISTRATION
General Considerations
• The dosage schedules listed below can be used as a general guide for the administration of Engystol® Injection Solution.
• Engystol® Injection Solution may be administered s.c., i.d., i.m., or i.v.
• The interval between injections is left to the discretion of the HCP, but should not exceed 1 ampule in 24 hours.
• Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard any unused ampule contents.
• Draw up required dose into syringe.
• Discard any unused ampule contents. Do not reuse ampule.
Standard Dosage:
Adults and children 12 years and older: 1 ml 1 to 3 times per 7 days.
Children 6 to 11 years: 0.7 ml 1 to 3 times per 7 days.
Children 2 to 5 years: 0.5 ml 1 to 3 times per 7 days.
Acute Dosage:
Adults and children 12 years and older: 1 ml daily, and then continue with standard dosage.
Children 6 to 11 years: 0.7 ml daily, and then continue with standard dosage.
Children 2 to 5 years: 0.5 ml daily, and then continue with standard dosage.
CONTRAINDICATIONS
Engystol® Injection Solution is contraindicated in patients with known hypersensitivity to Engystol® or any of its ingredients.
OVERDOSAGE
No negative effects of an overdose have been reported and none are expected due to the homeopathic dilutions.
CLINICAL PHARMACOLOGY
Mechanism of Action
The exact mechanism of Engystol® Injection Solution is not fully understood.
Pharmacodynamics
Not applicable for homeopathic medicinal products.
| ENGYSTOL
cynanchum vincetoxicum root and sulfur injection |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - Heel Inc (102783016) |