GASTROGARD
-
omeprazole paste
Merial Limited
----------
GastroGard®(omeprazole) Oral Paste for Equine Ulcers
Oral Paste for Horses and Foals
- NADA 141-123, Approved by FDA
Caution
- Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
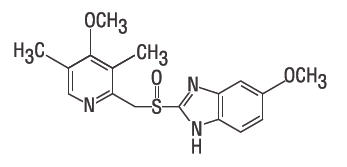
- Chemical name: 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl) methyl]sulfinyl]-1H-benzimidazole. Empirical formula: C17H19N3O3S. Molecular weight: 345.42. Structural formula:
How Supplied
- GastroGard (omeprazole) Paste for horses contains 37% w/w omeprazole and is available in an adjustable-dose syringe. Each syringe contains 2.28 g of omeprazole. Syringes are calibrated according to body weight and are available in boxes of 7 units or 72 units.
Storage Conditions
- Store at 68°F – 77°F (20-25°C). Excursions between 59°F – 86°F (15-30°C) are permitted.
Indications
- For treatment and prevention of recurrence of gastric ulcers in horses and foals 4 weeks of age and older.
Dosage Regimen
- For treatment of gastric ulcers, GastroGard Paste should be administered orally once-a-day for 4 weeks at the recommended dosage of 1.8 mg omeprazole/lb body weight (4 mg/kg). For the prevention of recurrence of gastric ulcers, continue treatment for at least an additional 4 weeks by administering GastroGard Paste at the recommended daily maintenance dose of 0.9 mg/lb (2 mg/kg).
Directions For Use
- GastroGard Paste for horses is recommended for use in horses and foals 4 weeks of age and older. The contents of one syringe will dose a 1250 lb (568 kg) horse at the rate of 1.8 mg omeprazole/lb body weight (4 mg/kg). For treatment of gastric ulcers, each weight marking on the syringe plunger will deliver sufficient omeprazole to treat 250 lb (114 kg) body weight. For prevention of recurrence of gastric ulcers, each weight marking will deliver sufficient omeprazole to dose 500 lb (227 kg) body weight.
- To deliver GastroGard Paste at the treatment dose rate of 1.8 mg omeprazole/lb body weight (4 mg/kg), set the syringe plunger to the appropriate weight marking according to the horse's weight in pounds.
- To deliver GastroGard Paste at the dose rate of 0.9 mg/lb (2 mg/kg) to prevent recurrence of ulcers, set the syringe plunger to the weight marking corresponding to half of the horse's weight in pounds.
- To set the syringe plunger, unlock the knurled ring by rotating it 1/4 turn. Slide the knurled ring along the plunger shaft so that the side nearest the barrel is at the appropriate notch. Rotate the plunger ring 1/4 turn to lock it in place and ensure it is locked. Make sure the horse's mouth contains no feed. Remove the cover from the tip of the syringe, and insert the syringe into the horse's mouth at the interdental space. Depress the plunger until stopped by the knurled ring. The dose should be deposited on the back of the tongue or deep into the cheek pouch. Care should be taken to ensure that the horse consumes the complete dose. Treated animals should be observed briefly after administration to ensure that part of the dose is not lost or rejected. If any of the dose is lost, redosing is recommended.
- If, after dosing, the syringe is not completely empty, it may be reused on following days until emptied. Replace the cap after each use.
Warning
- Do not use in horses intended for human consumption. Keep this and all drugs out of the reach of children. In case of ingestion, contact a physician. Physicians may contact a poison control center for advice concerning accidental ingestion.
Adverse Reactions
- In efficacy trials, when the drug was administered at 1.8 mg omeprazole/lb (4 mg/kg) body weight daily for 28 days and 0.9 mg omeprazole/lb (2 mg/kg) body weight daily for 30 additional days, no adverse reactions were observed.
Precautions
- The safety of GastroGard Paste has not been determined in pregnant or lactating mares.
Clinical Pharmacology
- Mechanism of Action: Omeprazole is a gastric acid pump inhibitor that regulates the final step in hydrogen ion production and blocks gastric acid secretion regardless of the stimulus. Omeprazole irreversibly binds to the gastric parietal cell's H+, K+ ATPase enzyme which pumps hydrogen ions into the lumen of the stomach in exchange for potassium ions. Since omeprazole accumulates in the cell cannaliculi and is irreversibly bound to the effect site, the plasma concentration at steady state is not directly related to the amount that is bound to the enzyme. The relationship between omeprazole action and plasma concentration is a function of the rate-limiting process of H+, K+ ATPase activity/turnover. Once all of the enzyme becomes bound, acid secretion resumes only after new H+, K+ ATPase is synthesized in the parietal cell (i.e., the rate of new enzyme synthesis exceeds the rate of inhibition).
- Pharmacodynamics: In a study of pharmacodynamic effects using horses with gastric cannulae, secretion of gastric acid was inhibited in horses given 4 mg omeprazole/kg/day. After the expected maximum suppression of gastric acid secretion was reached (5 days), the actual secretion of gastric acid was reduced by 99%, 95% and 90% at 8, 16, and 24 hours, respectively.
- Pharmacokinetics: In a pharmacokinetic study involving thirteen healthy, mixed breed horses (8 female, 5 male) receiving multiple doses of omeprazole paste (1.8 mg/lb once daily for fifteen days) in either a fed or fasted state, there was no evidence of drug accumulation in the plasma when comparing the extent of systemic exposure (AUC0-∞). When comparing the individual bioavailability data (AUC0-∞, Cmax, and Tmax measurements) across the study days, there was great inter- and intrasubject variability in the rate and extent of product absorption. Also, the extent of omeprazole absorption in horses was reduced by approximately 67% in the presence of food. This is evidenced by the observation that the mean AUC0-∞ values measured during the fifth day of omeprazole therapy when the animals were fasted for 24 hours was approximately three times greater than the AUC estimated after the first and fifteenth doses when the horses were fed hay ad libitum and sweet feed (grain) twice daily. Prandial status did not affect the rate of drug elimination. The terminal half-life estimates (N=38) ranged from approximately one-half to eight hours.
Efficacy
- Dose Confirmation: GastroGard (omeprazole) Paste, administered to provide omeprazole at 1.8 mg/lb (4 mg/kg) daily for 28 days, effectively healed or reduced the severity of gastric ulcers in 92% of omeprazole-treated horses. In comparison, 32% of controls exhibited healed or less severe ulcers. Horses enrolled in this study were healthy animals confirmed to have gastric ulcers by gastroscopy. Subsequent daily administration of GastroGard Paste to provide omeprazole at 0.9 mg/lb (2 mg/kg) for 30 days prevented recurrence of gastric ulcers in 84% of treated horses, whereas ulcers recurred or became more severe in horses removed from omeprazole treatment.
- Clinical Field Trials: GastroGard Paste administered at 1.8 mg/lb (4 mg/kg) daily for 28 days healed or reduced the severity of gastric ulcers in 99% of omeprazole-treated horses. In comparison, 32.4% of control horses had healed ulcers or ulcers which were reduced in severity. These trials included horses of various breeds and under different management conditions, and included horses in race or show training, pleasure horses, and foals as young as one month. Horses enrolled in the efficacy trials were healthy animals confirmed to have gastric ulcers by gastroscopy. In these field trials, horses readily accepted GastroGard Paste. There were no drug related adverse reactions. In the clinical trials, GastroGard Paste was used concomitantly with other therapies, which included: anthelmintics, antibiotics, non-steroidal and steroidal anti-inflammatory agents, diuretics, tranquilizers and vaccines.
-
Diagnostic and Management Considerations: The following clinical signs may be associated with gastric ulceration in adult horses: inappetance or decreased appetite, recurrent colic, intermittent loose stools or chronic diarrhea, poor hair coat, poor body condition, or poor performance. Clinical signs in foals may include: bruxism (grinding of teeth), excessive salivation, colic, cranial abdominal tenderness, anorexia, diarrhea, sternal recumbency or weakness. A more accurate diagnosis of gastric ulceration in horses and foals may be made if ulcers are visualized directly by endoscopic examination of the gastric mucosa.
Gastric ulcers may recur in horses if therapy to prevent recurrence is not administered after the initial treatment is completed. Use GastroGard Paste at 0.9 mg omeprazole/lb body weight (2 mg/kg) for control of gastric ulcers following treatment. The safety of administration of GastroGard Paste for longer than 91 days has not been determined.
Maximal acid suppression occurs after three to five days of treatment with omeprazole.
Safety
- GastroGard Paste was well tolerated in the following controlled efficacy and safety studies.
- In field trials involving 139 horses, including foals as young as one month of age, no adverse reactions attributable to omeprazole treatment were noted.
- In a placebo controlled adult horse safety study, horses received 20 mg/kg/day omeprazole (5x the recommended dose) for 90 days. No treatment related adverse effects were observed.
- In a placebo controlled tolerance study, adult horses were treated with GastroGard Paste at a dosage of 40 mg/kg/day (10x the recommended dose) for 21 days. No treatment related adverse effects were observed.
- A placebo controlled foal safety study evaluated the safety of omeprazole at doses of 4, 12 or 20 mg/kg (1, 3 or 5x) once daily for 91 days. Foals ranged in age from 66 to 110 days at study initiation. Gamma glutamyltransferase (GGT) levels were significantly elevated in horses treated at exaggerated doses of 20 mg/kg (5x the recommended dose). Mean stomach to body weight ratio was higher for foals in the 3x and 5x groups than for controls; however, no abnormalities of the stomach were evident on histological examination.
Reproductive Safety
- In a male reproductive safety study, 10 stallions received GastroGard Paste at 12 mg/kg/day (3x the recommended dose) for 70 days. No treatment related adverse effects on semen quality or breeding behavior were observed. A safety study in breeding mares has not been conducted.
For More Information
- Please call 1-888-637-4251.
Marketed by: Merial LLC, Duluth, GA 30096-4640, U.S.A.
Made in Brazil
US Patent: 4255431 and 5708017
®GastroGard is a registered trademark of the AstraZeneca
Group of Companies.
©2009 Merial.
All rights reserved. Rev. 02-2009
PRINCIPAL DISPLAY PANEL - Label
GastroGard®
(omeprazole)
NADA 141-123, Approved by FDA
Caution: Federal (USA) law restricts this drug to use by or on the
order of a licensed veterinarian.
Indications: For treatment and prevention of recurrence of gastric
ulcers in horses and foals 4 weeks of age and older.
Dosage Regimen: For oral use in horses only. For treatment of
gastric ulcers, GastroGard® (omeprazole) Paste should be administered
orally once-a-day for 4 weeks at the recommended dosage of 1.8 mg
omeprazole/lb body weight (4 mg/kg). For the prevention of
recurrence of gastric ulcers, continue treatment for at least an
additional 4 weeks by administering GastroGard (omeprazole) Paste at
the recommended daily maintenance dose of 0.9 mg/lb (2 mg/kg).
NET WT. 6.15 g (2.28 g of omeprazole)
READ PACKAGE INSERT FOR FURTHER
INSTRUCTIONS AND IMPORTANT INFORMATION.
Warning: Do not use in horses intended for human
consumption. Keep this and all drugs out of the reach of children.
Storage: Store at 68°F - 77°F (20-25°C). Excursions between
59°F - 86°F (15-30°C) are permitted.
Marketed by: Merial LLC, Duluth, GA 30096-4640, U.S.A.
Made in Brazil
Customer Assistance: For more information, please call
1-888-637-4251. US Patent: 4255431 and 5708017
®GastroGard is a registered trademark of the AstraZeneca
Group of Companies. ©2009 Merial. All rights reserved.
1824/01 / 36611, Rev. 02-2009
LOT
EXP

PRINCIPAL DISPLAY PANEL - 7 Syrings Carton
GastroGard®
(omeprazole) Oral Paste for Equine Ulcers
Improves and Heals Gastric Ulcers
Prevents Gastric Ulcer Recurrence
Once-a-Day Dosing
NADA 141-123, Approved by FDA
Contains 7 Syringes
Individually Wrapped
Product 3661101
MERIAL

PRINCIPAL DISPLAY PANEL - 72 Syringe Shipping Label
READ PACKAGE INSERT FOR FURTHER INSTRUCTIONS
AND IMPORTANT INFORMATION.
GastroGard®
(omeprazole) Oral Paste for Equine Ulcers
For Treatment and Prevention of
Recurrence of Equine Gastric Ulcers
CAUTION: Federal (USA) law restricts this drug to use by or
on the order of a licensed veterinarian.
Store at 68°F – 77°F (20-25°C).
Excursions between 59°F – 86°F (15-30°C) are permitted.
Lot No & Exp Date
Product 3661172
Marketed by:
Merial LLC,
Duluth, GA 30096-4640, U.S.A.
Made in Brazil
1829/01
36611
Rev. 02-2009
®GastroGard is a
registered trademark of
the AstraZeneca Group
of Companies.
©2009 Merial.
All rights reserved.
CONTENTS: 72 Syringes Individually Wrapped
MERIAL

| GASTROGARD
omeprazole paste |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NADA | NADA141123 | 03/16/1999 | |
| Labeler - Merial Limited (034393582) |