methocarbamol (Methocarbamol) tablet
[Danbury Pharmacal,Inc.]
DESCRIPTION
Methocarbamol is an aromatic glycerol ether and is a close chemical relative to mephenesin carbamate, the oldest and most extensively studied of the drugs in this class. The chemical name is 3-(o-Methoxyphenoxy)-1,2-propanediol 1-carbamate. The structural formula is represented below:
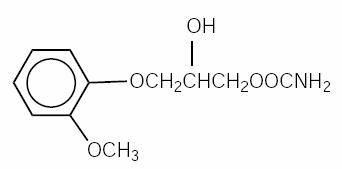
C11H15NO5 M.W. 241.24
Methocarbamol Tablets, USP 500 mg and 750 mg contain the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, sodium starch glycolate, pregelatinized starch and stearic acid.
CLINICAL PHARMACOLOGY
The mechanism of action of methocarbamol in humans has not been established, but may be due to central nervous system depression. It has no direct action on the contractile mechanism of striated muscle, the motor end plate or the nerve fiber.
INDICATIONS AND USAGE
Methocarbamol Tablets are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Methocarbamol does not directly relax tense skeletal muscles in man.
CONTRAINDICATIONS
Methocarbamol Tablets are contraindicated in patients hypersensitive to any of the ingredients.
WARNINGS
Since methocarbamol may possess a central nervous system depressant effect, patients receiving methocarbamol tablets should be cautioned about combined effects with alcohol and other CNS depressants.
Safe use of methocarbamol has not been established with regard to possible adverse effects upon fetal development. Therefore, methocarbamol tablets should not be used in women who are, or may become, pregnant and particularly during early pregnancy unless in the judgment of the physician, the potential benefits outweigh the possible hazards.
PRECAUTIONS
Safety and effectiveness in children below the age of twelve (12) years have not been established.
It is not known whether this drug is excreted in human milk. As a general rule, nursing should not be undertaken while a patient is on a drug since many drugs are excreted in human milk. Methocarbamol may cause a color interference in certain screening tests for 5-hydroxyindoleacetic acid (5-HIAA) and vanillylmandelic acid (VMA).
ADVERSE REACTIONS
Lightheadedness, dizziness, drowsiness, nausea, allergic manifestations such as urticaria, pruritus, rash, conjunctivitis with nasal congestion, blurred vision, headache, fever.
DOSAGE AND ADMINISTRATION
500 mg - Adults:
initial dosage, 3 tablets q.i.d.; maintenance dosage, 2 tablets q.i.d.
750 mg - Adults:
initial dosage, 2 tablets q.i.d.; maintenance dosage, 1 tablet q.4h, or 2 tablets t.i.d.
Six grams a day are recommended for the first 48 to 72 hours of treatment. (For severe conditions 8 grams a day may be administered.) Thereafter, the dosage can usually be reduced to approximately 4 grams a day.
HOW SUPPLIED
Methocarbamol Tablets, USP 500 mg are scored, round, white tablets imprinted “DAN DAN” and “5381” supplied in bottles of 100, 500 and 1000.
Methocarbamol Tablets, USP 750 mg are scored, capsule shaped, white tablets imprinted “DAN DAN” and “5382” supplied in bottles of 100, 500 and 1000.
Dispense in a tight container with a child-resistant closure.
Store at controlled room temperature 15°-30°C (59°-86°F).
Rx Only
Manufactured by:
DANBURY PHARMACAL, INC.
Danbury, CT 06810
| Methocarbamol (Methocarbamol) | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Methocarbamol (Methocarbamol) | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
Revised: 03/2006Danbury Pharmacal,Inc.