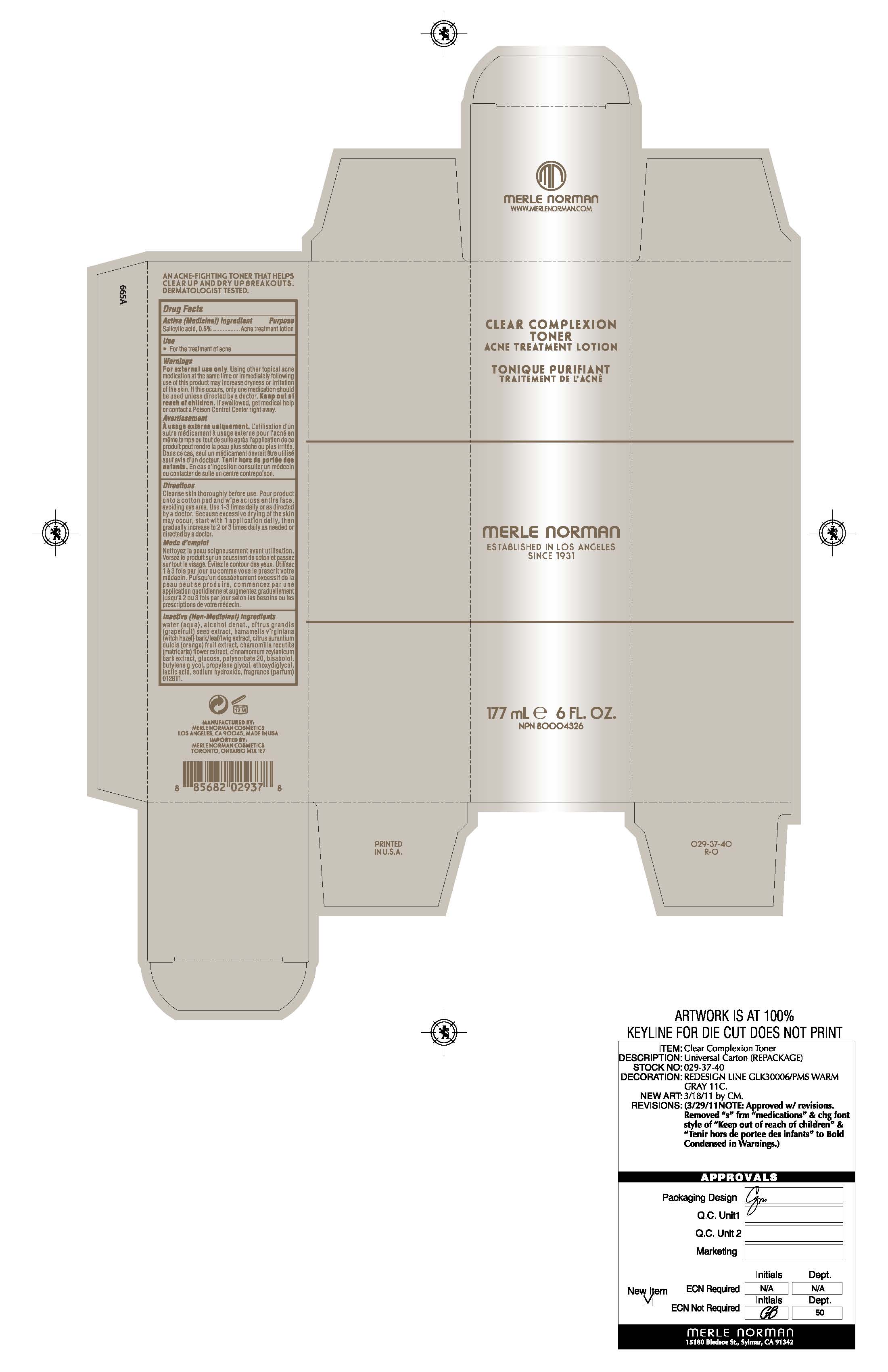
CLEAR COMPLEXION TONER MERLE NORMAN - salicylic acid lotion
Merle Norman Cosmetics, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Merle Norman Clear Complexion Toner
active ingredients purpose
Salicylic Acid 0.5% Acne treatment lotion
using other topical acne medication at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor
cleanse skin thoroughly before use. pour product onto a cotton pad and wipe across entire face avoiding eye area. use 1-3 times daily or as directed by a doctor. because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily as needed or directed by a doctor.
water (aqua) , alcohol denat., citrus grandis (grapefruit) seed extract , hamamelis virginiana (witch hazel) bark/leaf/twig extract, citrus aurantium dulcis (orange) fruit extract, chamomilla recutita (matricaria) flower extract, cinnamomum zeylanicum bark extract, glucose, polysorbate 20, bisabolol, butylene glycol, propylene glycol, ethoxydiglycol, lactic acid, sodium hydroxide , fragrance (parfum)
| CLEAR COMPLEXION TONER
MERLE NORMAN
salicylic acid lotion |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Merle Norman Cosmetics, Inc (008479388) |
| Registrant - Merle Norman Cosmetics, Inc (008479388) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Merle Norman Cosmetics, Inc | 008479388 | manufacture(57627-102) | |