DONOR PREP KIT- isopropyl alcohol and iodine
CareFusion 213 LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
Frepp
- •
- for the preparation of the patient's skin prior to injection
Sepp
- •
- patient preoperative skin preparation: Helps to reduce bacteria that potentially can cause skin infection
Warnings
For external use only
Flammable: Keep away from fire or flame
Do not use with electrocautery procedures
Do not use
Frepp
on patients with known allergies to isopropyl alcohol
Sepp
on patients with known allergies to ethyl alcohol or iodine
Directions
Frepp
- •
- locate the vein to be used
- •
- remove Frepp from kit. Hold in a horizontal position and pinch handle once to break ampule. Do not continue to squeeze handle.
- •
- place sponge on selected venipuncture site and depress once or twice to saturate sponge
- •
- scrub vigorously for at least 30 seconds
- •
- allow to dry
Sepp
- •
- remove Sepp. Hold in downward position and pinch center of Sepp to crush ampule.
- •
- use repeated back-and-forth strokes of the applicator for approximately 30 seconds or apply iodine tincture to venipuncture site starting at center and moving outward in concentric circles to periphery. Allow to dry.
- •
- proceed with collection of blood
- •
- discard unused components
Inactive ingredients
Frepp
- •
- USP purified water
Sepp
- •
- USP purified water
- •
- ethyl alcohol
- •
- sodium iodide USP
Package/Label Principal Display Panel
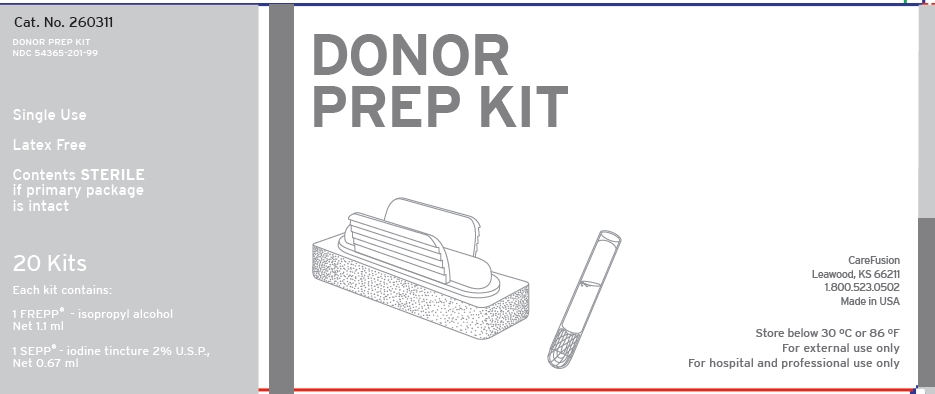
DONOR PREP KIT
NDC 54365-201-99
Single Use
Latex Free
Contents STERILE if primary package is intact
20 Kits
Each kit contains:
1 FREPP - isopropyl alcohol
Net 1.1 ml
1 SEPP - iodine tincture 2% USP
Net 0.67 ml
DONOR PREP KIT
CareFusion
Leawood, KS 66211
1.800.523.0502
Made in
Store below 30 °C or 86 °F
For external use only
For hospital and professional use only
| DONOR PREP KIT
isopropyl alcohol and iodine kit |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - CareFusion 213 LLC (826496312) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| CareFusion 213 LLC | 826496312 | ANALYSIS(54365-201), LABEL(54365-201), MANUFACTURE(54365-201), PACK(54365-201) | |