ALAVERT ALLERGY
-
loratadine tablet, orally disintegrating
Wyeth Consumer Healthcare
----------
Alavert Allergy (Original)Alavert Allergy (Fresh Mint)
Alavert Allergy (Bubble Gum)
Alavert Allergy (Citrus Burst)
(loratadine)
DRUG FACTS
ACTIVE INGREDIENT (IN EACH TABLET)
PURPOSE
USES
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
WARNINGS
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not use more than directed. Taking more than recommended may cause drowsiness.
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- tablet melts in mouth. Can be taken with or without water.
| Age | Dose |
|---|---|
| adults and children 6 years and over | 1 tablet daily; do not use more than 1 tablet daily |
| children under 6 | ask a doctor |
| consumers who have liver or kidney disease | ask a doctor |
OTHER INFORMATION
- Phenylketonurics: Contains Phenylalanine 8.4 mg per tablet
- store at 20-25ºC (68-77ºF)
- keep in a dry place
INACTIVE INGREDIENTS
Alavert Allergy Original Flavor
artificial & natural flavor, aspartame, citric acid, colloidal silicone dioxide, corn syrup solids, crospovidone, magnesium stearate, mannitol, microcrystalline cellulose, modified food starch, sodium bicarbonate
Alavert Allergy Fresh Mint
artificial & natural flavors, anhydrous citric acid, aspartame, colloidal silicone dioxide, corn syrup solids, crospovidone, magnesium stearate, mannitol, microcrystalline cellulose, modified food starch, sodium bicarbonate
Alavert Allergy Bubble Gum
anhydrous citric acid, artificial and natural flavor, ascorbic acid, aspartame, colloidal silicon dioxide, crospovidone, ferric oxide, magnesium stearate, maltodextrin, mannitol, microcrystalline cellulose, modified starch, sodium bicarbonate
Alavert Allergy Citrus Burst
anhydrous citric acid, aspartame, butylated hydroxyanisole, colloidal silicon dioxide, corn syrup solids, crospovidone, dextrin, ferric oxides, magnesium stearate, maltodextrin, mannitol, microcrystalline cellulose, modified food starch, natural & artificial flavors, sodium bicarbonate
QUESTIONS OR COMMENTS?
call weekdays from 9 AM to 5 PM EST at 1-800-ALAVERT (1-800-252-8378)
PRODUCT PACKAGING
The product packaging shown below represents a sample of that currently in use. Additional packaging may also be available.
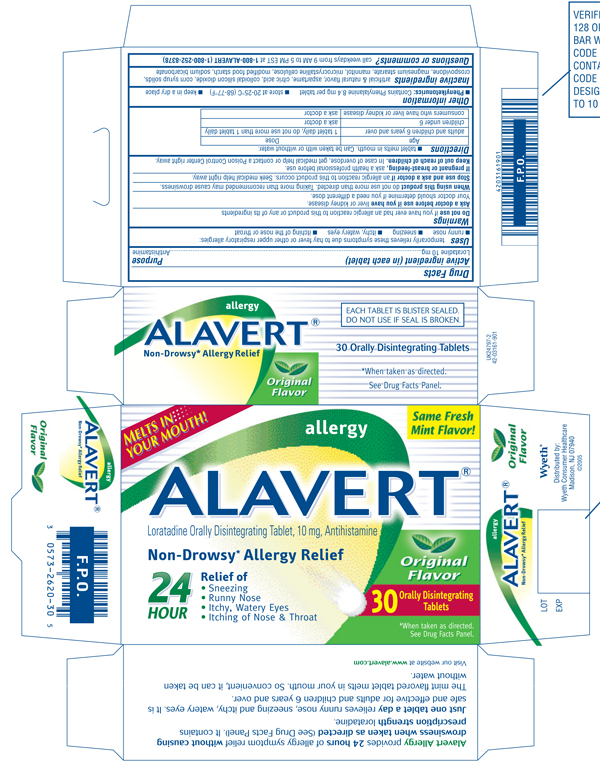
ALAVERT allergy Original Flavor
Loratadine Orally Disintegrating Tablet, 10 mg, Antihistamine
30 Orally Disintegrating Tablets
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itching of Nose & Throat
*When taken as directed. See Drug Facts Panel.
Alavert Allergy provides 24 hours of allergy symptom relief without causing drowsiness when taken as directed (See Drug Facts Panel). It contains prescription strength loratadine.
Just one tablet a day relieves runny nose, sneezing and itchy, watery eyes. It is safe and effective for adults and children 6 years and over.
The mint flavored tablet melts in your mouth. So convenient, it can be taken without water.
Visit our website at www.alavert.com
EACH TABLET IS BLISTER SEALED. DO NOT USE IF SEAL IS BROKEN.
ALAVERT allergy Bubble Gum Flavor
Loratadine Orally Disintegrating Tablet, 10 mg, Antihistamine
12 Orally Disintegrating Tablets
24 HOUR Non-Drowsy* Allergy Relief
- Itching of Nose & Throat
- Itchy, Watery Eyes
- Sneezing
- Runny Nose
*When taken as directed. See Drug Facts Panel.
Alavert Allergy provides 24 hours of allergy symptom relief without causing drowsiness when taken as directed (See Drug Facts Panel). It contains prescription strength loratadine. Just one tablet a day relieves runny nose, sneezing and itchy, watery eyes. It is safe and effective for adults and children 6 years and over. The Bubble Gum flavored tablet melts in your mouth. So convenient, it can be taken without water. Visit our website at www.alavert.com
Distributed by: Wyeth Consumer Healthcare, Madison, NJ 07940 U.S.A. ©2008 Wyeth
EACH TABLET IS BLISTER SEALED. DO NOT USE IF SEAL IS BROKEN.

Loratadine Orally Disintegrating Tablet, 10 mg, Antihistamine
12 Orally Disintegrating Tablets
24 HOUR Non-Drowsy* Allergy Relief
- Itching of Nose & Throat
- Itchy, Watery Eyes
- Sneezing
- Runny Nose
*When taken as directed. See Drug Facts Panel.
Alavert Allergy provides 24 hours of allergy symptom relief without causing drowsiness when taken as directed (See Drug Facts Panel). It contains prescription strength loratadine. Just one tablet a day relieves runny nose, sneezing and itchy, watery eyes. It is safe and effective for adults and children 6 years and over. The Citrus Burst flavored tablet melts in your mouth. So convenient, it can be taken without water. Visit our website at www.alavert.com
Distributed by: Wyeth Consumer Healthcare, Madison, NJ 07940 U.S.A. ©2008 Wyeth
EACH TABLET IS BLISTER SEALED. DO NOT USE IF SEAL IS BROKEN.

| ALAVERT ALLERGY
loratadine tablet, orally disintegrating |
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021375 | 12/19/2002 | |
| ALAVERT ALLERGY
loratadine tablet, orally disintegrating |
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021375 | 12/19/2002 | |
| ALAVERT ALLERGY
loratadine tablet, orally disintegrating |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021375 | 12/19/2002 | |
| ALAVERT ALLERGY
loratadine tablet, orally disintegrating |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021375 | 12/19/2002 | |
| Labeler - Wyeth Consumer Healthcare (828831730) |