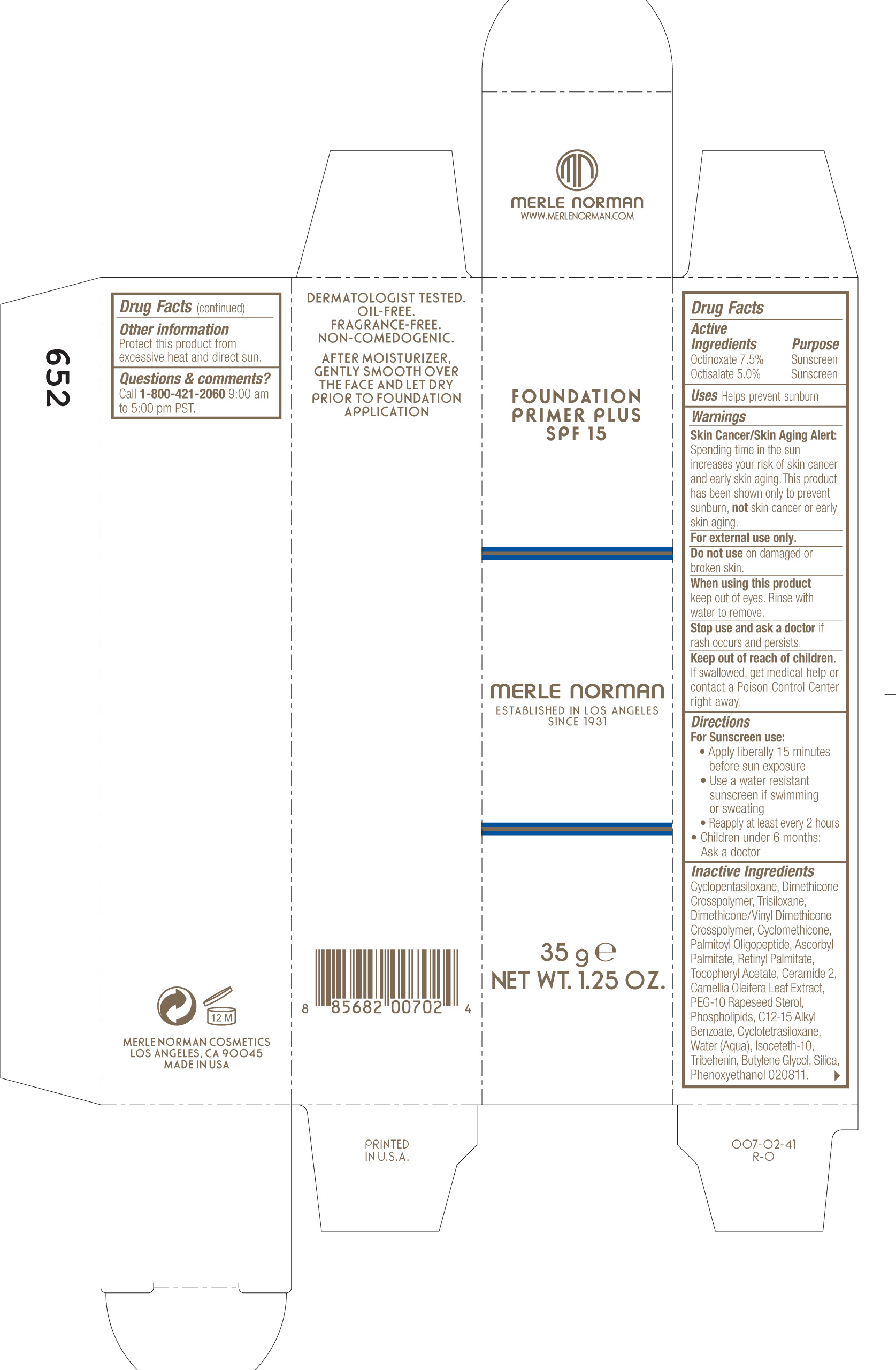
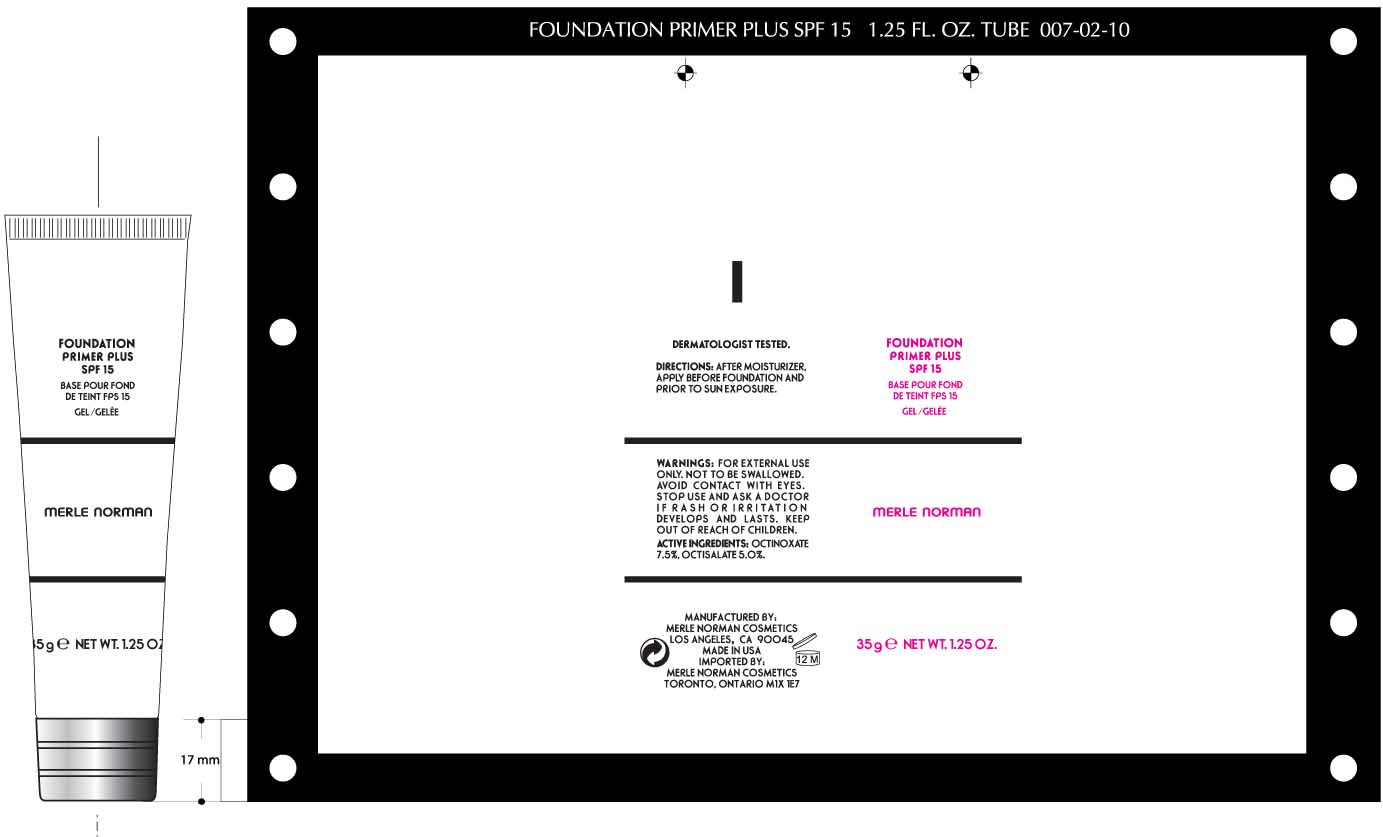
FOUNDATION PRIMER PLUS SPF 15 MERLE NORMAN- octinoxate and octisalate cream
Merle Norman Cosmetics, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Merle Norman Foundation Primer Plus SPF 15
Active ingredients purpose
OCTINOXATE 7.5% Sunscreen
OCTISALATE 5.0% Sunscreen
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
WARNINGS
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only.
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Directions
For Sunscreen use
· Apply liberally 15 minutes before sun exposure
· Use a water resistant sunscreen if swimming or sweating
· Reapply at least every 2 hours
· Children under 6 months: Ask a doctor
Cyclopentasiloxane, dimethicone crosspolymer, trisiloxane, dimethicone/ vinyl dimethicone crosspolymer, cyclomethicone , palmitoyl oligopeptide, ascorbyl palmitate, retinyl palmitate, tocopheryl acetate, ceramide 2, camellia oleifera leaf extract, peg-10 rapeseed sterol, phospholipids , C12-15 alkyl benzoate, cyclotetrasiloxane, water (aqua), isoceteth-10, tribehenin, butylene glycol, silica, phenoxyethanol
| FOUNDATION PRIMER PLUS SPF 15
MERLE NORMAN
octinoxate, octisalate cream |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Merle Norman Cosmetics, Inc (008479388) |
| Registrant - Merle Norman Cosmetics, Inc (008479388) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Merle Norman Cosmetics, Inc | 008479388 | manufacture(57627-108) | |