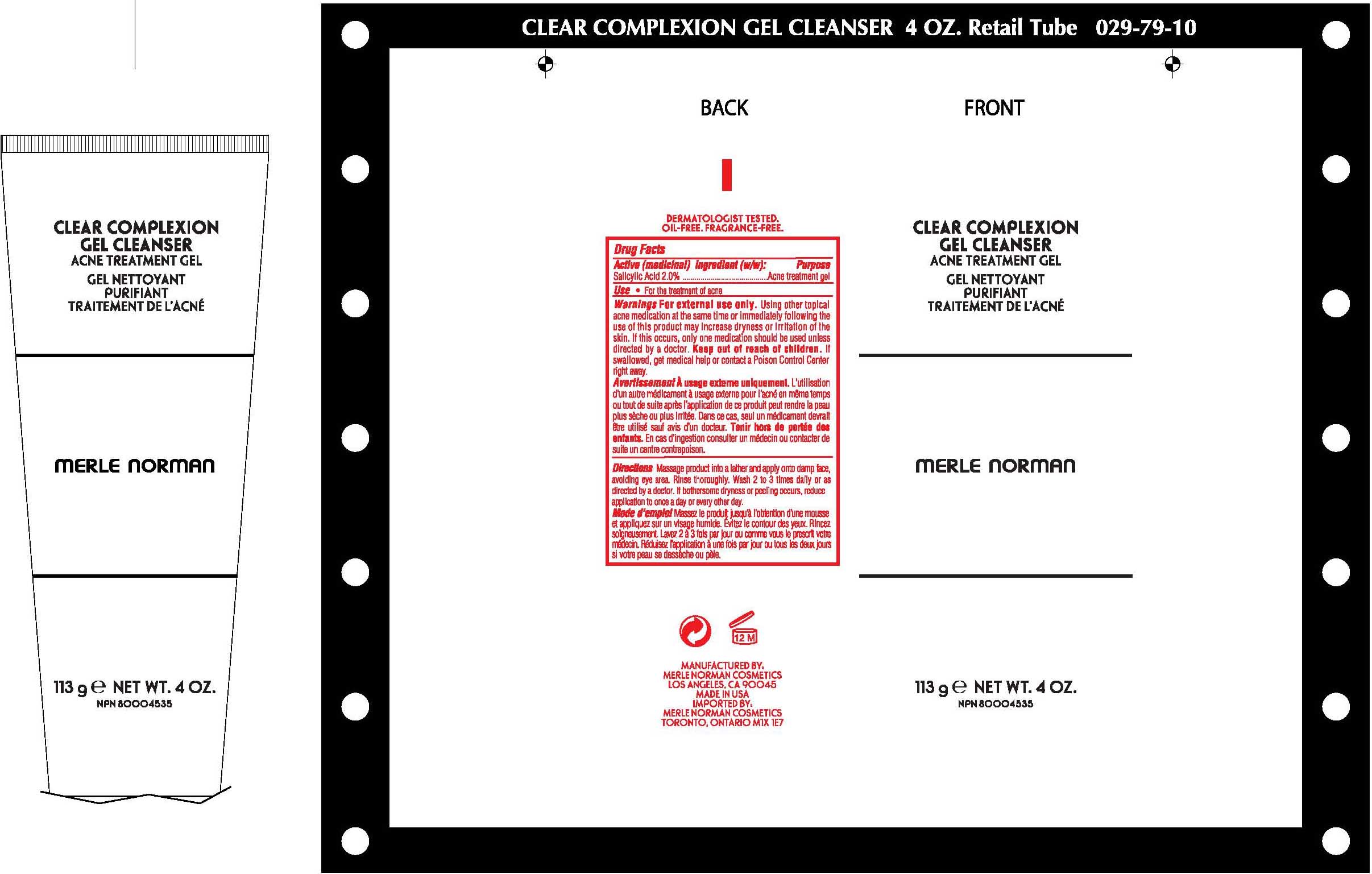
CLEAR COMPLEXION CLEANSER MERLE NORMAN- salicylic acid gel
Merle Norman
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Clear Complexion Gel Cleanser
Active (Medicinal) Ingredient Purpose
Salicylic acid, 2.0% Acne treatment gel
Using other topical acne medication at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
Direction
Massage product into a lather and and apply onto damp face, avoiding eye area. Rinse thoroughly. Wash 2 to 3 times daily or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Water (aqua), disodium Laureth sulfocinnate, sodium laureth sulfate, cocamidopropyl betaine, butylene glycol, peg-150 distearate, citrus grandis (grapefruit) seed extract, citrus aurantium dulcis (orange) fruit extract, chamomilla recutita (matrcaria) flower extract, hamamelis virginiana (which Hazel) bark/leaf/twig extrract, cinnamomum zeylanicum bark extract, bisabolol, panthenyl hydroxypropyl steardimonium chloride, tocopheryl acetate, retinyl palmitate, ascorbal palmitate, phospholipids, glucose, lactic acid, propylene glycol, ethoxydiglycol, ppg-2 hydroxyethyl coco/isostearamide, disodium edta, sodium hydroxide, sodium chloride, phenoxyethanol, fragrance(parfum)
| CLEAR COMPLEXION CLEANSER
MERLE NORMAN
salicylic acid gel |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Merle Norman (008479388) |
| Registrant - Merle Norman (008479388) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Merle Norman | 008479388 | manufacture(57627-103) | |