INFANTS ACETAMINOPHEN- acetaminophen suspension
Precision Dose Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
temporarily:
- reduces fever
- relieves minor aches and pains due to:
- the common cold
- flu
- headache
- sore throat
- toothache
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if your child takes more than 5 doses in 24 hours, which is the maximum daily amount with other drugs containing acetaminophen
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly
Do not use with any other product containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Directions
- use as directed per healthcare professional
- do not give more than directed (see Liver warning)
- dispense liquid slowly into child's mouth, toward inner cheek
- if needed, repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
- this product does not contain directions or complete warnings for adult use
Other Information
- store at 20° - 25°C (68° - 77°F)
- See individual label or shipper label for lot number and expiration date.
Inactive Ingredients
anhydrous citric acid, butylparaben, carboxymethylcellulose sodium, carrageenan, FD&C red no. 40, flavor, glycerin, high fructose corn syrup, hydroxyethyl cellulose, microcrystalline cellulose, propylene glycol, purified water, sodium benzoate, sorbitol solution
How Supplied
NDC 68094-581-58
0.4 mL per unit dose syringe
Fifty (50) unit dose syringes per shipper
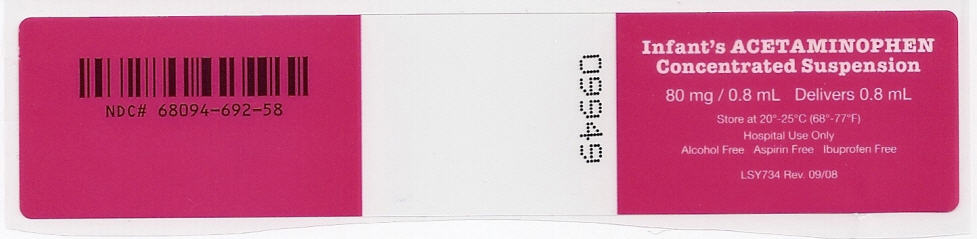
NDC 68094-692-58
0.8 mL per unit dose syringe
Fifty (50) unit dose syringes per shipper
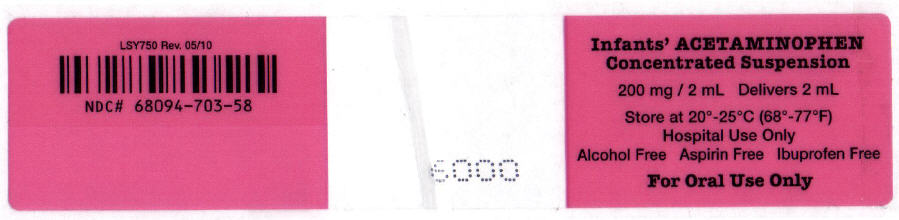
NDC 68094-703-58
2 mL per unit dose syringe
Fifty (50) unit dose syringes per shipper
Distributed By:
Perrigo Company
515 Eastern Avenue
Allegan, MI 49010
Packaged By:
Precision Dose, Inc.
722 Progressive Lane
S. Beloit, IL 61080
LI712 Rev. 05/10
| INFANTS ACETAMINOPHEN
acetaminophen suspension |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| INFANTS ACETAMINOPHEN
acetaminophen suspension |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Precision Dose Inc. (035886746) |