NOVOLIN N
-
insulin human injection, suspension
Novo Nordisk
----------
Novolin N NPH, Human Insulin Isophane Suspension (recombinant DNA origin) 100 units/mLPATIENT PACKAGE INSERT
Information for the Patient
Novolin® N
NPH, Human Insulin Isophane Suspension (recombinant DNA origin)
100 units/mL
Please read this leaflet carefully
WARNING
ANY CHANGE OF INSULIN SHOULD BE MADE CAUTIOUSLY AND ONLY UNDER MEDICAL SUPERVISION. CHANGES IN PURITY, STRENGTH, BRAND
(MANUFACTURER), TYPE (REGULAR, NPH, LENTE®,, ETC.), SPECIES (BEEF, PORK, BEEF-PORK, HUMAN), AND/OR METHOD OF MANUFACTURE (RECOMBINANT DNA VERSUS ANIMAL-SOURCE INSULIN) MAY RESULT IN THE NEED FOR A CHANGE IN DOSAGE.
SPECIAL CARE SHOULD BE TAKEN WHEN THE TRANSFER IS FROM A STANDARD BEEF OR MIXED SPECIES INSULIN TO A PURIFIED PORK OR HUMAN INSULIN. IF A DOSAGE ADJUSTMENT IS NEEDED, IT WILL USUALLY BECOME APPARENT EITHER IN THE FIRST FEW DAYS OR OVER A PERIOD OF SEVERAL WEEKS. ANY CHANGE IN TREATMENT SHOULD BE CAREFULLY MONITORED.
PLEASE READ THE SECTIONS "INSULIN REACTION AND SHOCK" AND "DIABETIC KETOACIDOSIS AND COMA" FOR SYMPTOMS OF HYPOGLYCEMIA (LOW BLOOD GLUCOSE) AND HYPERGLYCEMIA (HIGH BLOOD GLUCOSE).
INSULIN USE IN DIABETES
Your physician has explained that you have diabetes and that your treatment involves injections of insulin or insulin therapy combined with an oral antidiabetic medicine. Insulin is normally produced by the pancreas, a gland that lies behind the stomach. Without insulin, glucose (a simple sugar made from digested food) is trapped in the bloodstream and cannot enter the cells of the body. Some patients who don't make enough of their own insulin, or who cannot use the insulin they do make properly, must take insulin by injection in order to control their blood glucose levels.
Each case of diabetes is different and requires direct and continued medical supervision. Your physician has told you the type, strength and amount of insulin you should use and the time(s) at which you should inject it, and has also discussed with you a diet and exercise schedule. You should contact your physician if you experience any difficulties or if you have questions.
TYPES OF INSULINS
Standard and purified animal insulins as well as human insulins are available. Standard and purified insulins differ in their degree of purification and content of noninsulin material. Standard and purified insulins also vary in species source; they may be of beef, pork, or mixed beef and pork origin. Human insulin is identical in structure to the insulin produced by the human pancreas, and thus differs from animal insulins. Insulins vary in time of action and in strength; see PRODUCT DESCRIPTION and SYRINGES for additional information. Your physician has prescribed the insulin that is right for you; be sure you have purchased the correct insulin and check it carefully before you use it.
PRODUCT DESCRIPTION
This vial contains Novolin® N commonly known as NPH, Human Insulin Isophane Suspension (recombinant DNA origin). The concentration of this product is 100 units of insulin per milliliter. It is a cloudy or milky suspension of human insulin with protamine and zinc. The insulin substance (the cloudy material) settles at the bottom of the vial, therefore, the vial must be gently agitated or rotated so that the contents are uniformly mixed before a dose is withdrawn. Novolin N has an intermediate duration of action. The effect of Novolin N begins approximately 1½ hours after injection. The effect is maximal between 4 and 12 hours. The full duration of action may last up to 24 hours after injection.
The time course of action of any insulin may vary considerably in different individuals, or at different times in the same individual. Because of this variation, the time periods listed here should be considered as general guidance only.
This human insulin (recombinant DNA origin) is structurally identical to the insulin produced by the human pancreas. This human insulin is produced by recombinant DNA technology utilizing Saccharomyces cerevisiae (bakers' yeast) as the production organism.
STORAGE
Insulin should be stored in a cold place(36°-46°F/2°-8°C), preferably in a refrigerator, but not in the freezing compartment. Do not let it freeze. Keep the insulin vial in its carton so that it will stay clean and protected from light. If refrigeration is not possible, the bottle of insulin which you are currently using can be kept unrefrigerated as long as it is kept as cool as possible and away from heat and sunlight.
Never use Novolin N if the precipitate (the white deposit at the bottom of the vial) has become lumpy or granular in appearance or has formed a deposit of solid particles on the wall of the vial. This insulin should not be used if the liquid in the vial remains clear after the vial has been gently agitated.
Never use insulin after the expiration date which is printed on the vial label and carton.
SYRINGES
Use the Correct Syringe
Doses of insulin are measured in units. Some insulins are available in two strengths: U-100 and U-40. One milliliter (mL) of U-100 contains 100 units of insulin. One milliliter (mL) of U-40 contains 40 units of insulin. Be sure to use the proper syringe for the strength of the insulin prescribed for you. Syringes are clearly marked "For use with U-100 insulin" or "For use with U-40 insulin". Low dose U-100 syringes are also available. Failure to use the proper syringes can lead to mistakes in dosage.
Novo Nordisk insulin vials are intended for use with standard insulin syringes. Novo Nordisk has not evaluated the use of these vials with other devices for insulin delivery or with devices intended to aid in giving injections. Consult your doctor and the manufacturer of these devices before use with this product.
Disposable Syringes
Disposable syringes and needles require no sterilization provided the package is intact.
They should be used only once and discarded.
Reusable Syringes
Reusable syringes and needles must be sterilized before each use.
1. Boil the syringe parts and needles in a pan of water for at least five minutes. Keep a special pan for this purpose. Heavily chlorinated water should not be used; distilled water is preferable.
If boiling is not possible, the syringe parts and needles may be sterilized by immersion in 70% ethyl alcohol or 91% isopropyl alcohol for at least five minutes. Do not use bathing, rubbing or medicated alcohol for sterilization.
2. Assemble the syringe and fit the needle on the tip of the syringe being careful not to touch the surface of the plunger or needle.
3. Push the plunger in and out several times until the water (or alcohol) has been completely expelled. (The syringe should be thoroughly dried before its use.)
IMPORTANT
Failure to comply with the above and following antiseptic measures may lead to infections at the injection site.
PREPARING THE INJECTION
1. Clean your hands and the injection site with soap and water or with alcohol. Wipe the rubber stopper with an alcohol swab. (Note: Remove the tamper-resistant cap at first use. If the cap has already been removed, do not use this product, return it to your pharmacy.)
2. For insulin suspensions, roll the vial of insulin gently in your hands to mix it. Vigorous shaking immediately before the dose is drawn into the syringe may result in the formation of bubbles or froth which could cause dosage errors.
3. Pull back the plunger until the black tip reaches the marking for the number of units you will inject.
4. Push the needle through the rubber stopper into the vial.
5. Push the plunger all the way in. This inserts air into the bottle.
6. Turn the vial and syringe upside down and slowly pull the plunger back to a few units beyond the correct dose.
7. If there are air bubbles flick the syringe firmly with your finger to raise the air bubbles to the needle, then slowly push the plunger to the correct unit marking.
8. Lift the vial off the syringe.
GIVING THE INJECTION
1. The following areas are suitable for subcutaneous insulin injection: thighs, upper arms, buttocks, abdomen. Do not change areas without consulting your physician. The actual point of injection should be changed each time; injection sites should be about an inch apart.
2. The injection site should be clean and dry. Pinch up skin area to be injected and hold it firmly.
3. Hold the syringe like a pencil and push the needle quickly and firmly into the pinched-up area.
Release the skin and push plunger all the way in to inject insulin beneath the skin. To ensure that all the insulin is injected keep the needle in the skin for several seconds after injection with your finger on the plunger. Do not inject into a muscle unless your physician has advised it. You should never inject insulin into a vein.
5. Remove needle. If slight bleeding occurs, press lightly with a dry cotton swab for a few seconds - do not rub.
Note: The dose should be injected over 2-4 seconds. Preparations of insulin suspensions which are injected slowly may clog the tip of the needle, resulting in an inability to complete the injection. Syringe plugging does not occur when the drug is injected more rapidly. Use the injection technique recommended by your physician.
MIXING TWO TYPES OF INSULIN
Different insulins should be mixed only under instruction from a physician. Hypodermic syringes may vary in the amount of space between the bottom line and the needle ("dead space"), so if you are mixing two types of insulin be sure to discuss any change in the model and brand of syringe you are using with your physician or pharmacist. When you are mixing two types of insulin, always draw the Regular (clear) insulin into the syringe first.
USAGE IN PREGNANCY
It is particularly important to maintain good control of your diabetes during pregnancy and special attention must be paid to your diet, exercise and insulin regimens. If you are pregnant or nursing a baby, consult your physician or nurse educator.
INSULIN REACTION AND SHOCK
Insulin reaction "hypoglycemia" occurs when the blood glucose falls very low. This can happen if you take too much insulin, miss or delay a meal, exercise more than usual or work too hard without eating, or become ill (especially with vomiting or fever). Hypoglycemia can also happen if you combine insulin therapy and other medications that lower blood glucose, such as oral antidiabetic agents or other prescription and over-the-counter drugs. The first symptoms of an insulin reaction usually come on suddenly. They may include a cold sweat, fatigue, nervousness or shakiness, rapid heartbeat, or nausea. Personality change or confusion may also occur. If you drink or eat something right away (a glass of milk or orange juice, or several sugar candies), you can often stop the progression of symptoms. If symptoms persist, call your physician - an insulin reaction can lead to unconsciousness. If a reaction results in loss of consciousness, emergency medical care should be obtained immediately. If you have had repeated reactions or if an insulin reaction has led to a loss of consciousness, contact your physician. Severe hypoglycemia can result in temporary or permanent impairment of brain function and death.
In certain cases, the nature and intensity of the warning symptoms of hypoglycemia may change. A few patients have reported that after being transferred to human insulin, the early warning symptoms of hypoglycemia were less pronounced than they had been with animal-source insulin.
DIABETIC KETOACIDOSIS AND COMA
Diabetic ketoacidosis may develop if your body has too little insulin. The most common causes are acute illness or infection or failure to take enough insulin by injection. If you are ill you should check your urine for ketones. The symptoms of diabetic ketoacidosis usually come on gradually, over a period of hours or days, and include a drowsy feeling, flushed face, thirst and loss of appetite. Notify your physician right away if the urine test is positive for ketones (acetone) or if you have any of these symptoms. Fast, heavy breathing and rapid pulse are more severe symptoms and you should have medical attention right away. Severe, sustained hyperglycemia may result in diabetic coma and death.
ADVERSE REACTIONS
A few people with diabetes develop red, swollen and itchy skin where the insulin has been injected. This is called a "local reaction" and it may occur if the injection is not properly made, if the skin is sensitive to the cleansing solution, or if you are allergic to the insulin being used. If you have a local reaction, tell your physician.
Generalized insulin allergy occurs rarely, but when it does it may cause a serious reaction, including skin rash over the body, shortness of breath, fast pulse, sweating, and a drop in blood pressure. If any of these symptoms develop, you should seek emergency medical care.
If severe allergic reactions to insulin have occurred (i.e., generalized rash, swelling or breathing difficulties) you should be skin-tested with each new insulin preparation before it is used.
IMPORTANT NOTES
1. A change in the type, strength, species or purity of insulin could require a dosage adjustment. Any change in insulin should be made under medical supervision.
2. You may have learned how to test your urine or your blood for glucose. It is important to do these tests regularly and to record the results for review with your physician or nurse educator.
3. If you have an acute illness, especially with vomiting or fever, continue taking your insulin. If possible, stay on your regular diet. If you have trouble eating, drink fruit juices, regular soft drinks, or clear soups; if you can, eat small amounts of bland foods. Test your urine for glucose and ketones and, if possible, test your blood glucose. Note the results and contact your physician for possible insulin dose adjustment. If you have severe and prolonged vomiting, seek emergency medical care.
4. You should always carry identification which states that you have diabetes.
5. Always ask your physician or pharmacist before taking any drug.
Always consult your physician if you have any questions about your condition or the use of insulin.
Helpful information for people with diabetes is published by American Diabetes Association, 1660 Duke Street, Alexandria, VA 22314
Novo Nordisk®, Novolin®, and Lente®, are trademarks owned by Novo Nordisk A/S
For information contact Novo Nordisk Inc., Princeton, NJ 08540
Call 1-800-727-6500 for additional information
www.novonordisk-us.com
Manufactured by: Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
Printed in USA
Date of issue: December 2005
Information for the Patient
Novolin® N PenFill®
NPH, Human Insulin Isophane Suspension
(recombinant DNA origin)
3 mL Disposable Cartridge
(300 units per cartridge)
100 units/mL (U-100)
Please read this leaflet carefully before using this product.
Please note the special directions under PREPARING THE INJECTION.
Novolin® N PenFill® 3 mL is designed for use with Novo Nordisk 3 mL PenFill® cartridge compatible insulin delivery devices, with or without the addition of a NovoPen® 3 PenMate®, and NovoFine® disposable needles.
PenFill® cartridge is for single-person use only. See IMPORTANT NOTES section.
WARNING
ANY CHANGE OF INSULIN SHOULD BE MADE CAUTIOUSLY AND ONLY UNDER MEDICAL SUPERVISION. CHANGES IN PURITY, STRENGTH, BRAND (MANUFACTURER), TYPE (REGULAR, NPH, LENTE®, ETC.), SPECIES (BEEF, PORK, BEEF-PORK, HUMAN), AND/OR METHOD OF MANUFACTURE (RECOMBINANT DNA VERSUS ANIMAL-SOURCE INSULIN) MAY RESULT IN THE NEED FOR A CHANGE IN DOSAGE.
SPECIAL CARE SHOULD BE TAKEN WHEN THE TRANSFER IS FROM A STANDARD BEEF OR MIXED SPECIES INSULIN TO A PURIFIED PORK OR HUMAN INSULIN. IF A DOSAGE ADJUSTMENT IS NEEDED, IT WILL USUALLY BECOME APPARENT EITHER IN THE FIRST FEW DAYS OR OVER A PERIOD OF SEVERAL WEEKS. ANY CHANGE IN TREATMENT SHOULD BE CAREFULLY MONITORED.
PLEASE READ THE SECTIONS “INSULIN REACTION AND SHOCK” AND “DIABETIC KETOACIDOSIS AND COMA” FOR SYMPTOMS OF HYPOGLYCEMIA (LOW BLOOD GLUCOSE) AND HYPERGLYCEMIA (HIGH BLOOD GLUCOSE).
INSULIN USE IN DIABETES
Your physician has explained that you have diabetes and that your treatment involves injections of insulin or insulin therapy combined with an oral antidiabetic medicine. Insulin is normally produced by the pancreas, a gland that lies behind the stomach. Without insulin, glucose (a simple sugar made from digested food) is trapped in the bloodstream and cannot enter the cells of the body. Some patients who don’t make enough of their own insulin, or who cannot use the insulin they do make properly, must take insulin by injection in order to control their blood glucose levels.
Each case of diabetes is different and requires direct and continued medical supervision. Your physician has told you the type, strength and amount of insulin you should use and the time(s) at which you should inject it, and has also discussed with you a diet and exercise schedule. You should contact your physician if you experience any difficulties or if you have questions.
TYPES OF INSULINS
Standard and purified animal insulins as well as human insulins are available. Standard and purified insulins differ in their degree of purification and content of noninsulin material. Standard and purified insulins also vary in species source; they may be of beef, pork, or mixed beef and pork origin. Human insulin is identical in structure to the insulin produced by the human pancreas, and thus differs from animal insulins. Insulins vary in time of action; see PRODUCT DESCRIPTION for additional information. Your physician has prescribed the insulin that is right for you; be sure you have purchased the correct insulin and check it carefully before you use it.
PRODUCT DESCRIPTION
This package contains five (5) Novolin® N PenFill® 3 mL cartridges. Novolin N is commonly known as NPH, Human Insulin Isophane Suspension (recombinant DNA origin). The concentration of this product is 100 units of insulin per milliliter. It is a cloudy or milky suspension of human insulin with protamine and zinc. The insulin substance (the cloudy material) settles at the bottom of the cartridge, therefore, the cartridge must be rotated up and down as described under PREPARING THE INJECTION so that the contents are uniformly mixed before a dose is given. Novolin N has an intermediate duration of action. The effect of Novolin N begins approximately 1½ hours after injection. The effect is maximal between 4 and 12 hours. The full duration of action may last up to 24 hours after injection.
The time course of action of any insulin may vary considerably in different individuals, or at different times in the same individual. Because of this variation, the time periods listed here should be considered as general guidance only.
This human insulin (recombinant DNA origin) is structurally identical to the insulin produced by the human pancreas. This human insulin is produced by recombinant DNA technology utilizing Saccharomyces cerevisiae (bakers’ yeast) as the production organism.
INSULIN DELIVERY SYSTEMS
These Novolin N PenFill 3 mL cartridges are designed for use with Novo Nordisk® 3 mL PenFill cartridge compatible insulin delivery devices, with or without the addition of a NovoPen® 3 PenMate®, and NovoFine® disposable needles.
STORAGE
Insulin should be stored in a cold (36° - 46°F [2° - 8°C]) place, preferably in a refrigerator, but not in the freezer. Do not let it freeze. Keep Novolin N PenFill cartridge in the carton so that they will stay clean and protected from light. The Novolin N PenFill cartridge that you are currently using should not be refrigerated but should be kept as cool as possible (below 86°F [30°C]) and away from direct heat and light. Unrefrigerated Novolin N PenFill cartridges must be discarded 14 days after the first use, even if they still contain Novolin N insulin. Never use PenFill cartridges after the expiration date which is printed on the label and carton.
Never use any Novolin N PenFill cartridge if the precipitate (the white deposit) has become lumpy or granular in appearance or has formed a deposit of solid particles on the wall of the cartridge. This insulin should not be used if the liquid in the cartridge remains clear after it has been mixed.
IMPORTANT
Failure to follow the antiseptic measures listed below may lead to infections at the injection site.
– Disposable needles are for single use; they should be used only once and destroyed.
– Clean your hands and the injection site with soap and water or with alcohol.
– Wipe the rubber stopper on the insulin cartridge with an alcohol swab.
PREPARING THE INJECTION
Never place a single-use disposable needle on your insulin delivery device until you are ready to give an injection, and remove it immediately after the injection. If the needle is not removed, some liquid may be expelled from the cartridge causing a change in the insulin concentration (strength).
The cloudy material in an insulin suspension will settle to the bottom of the cartridge, so the contents must be mixed before injection. These Novolin PenFill cartridges contain a glass ball to aid mixing.
When using a new cartridge, turn the cartridge up and down between positions A and B - See Figure 1. Do this at least 10 times until the liquid appears uniformly white and cloudy.
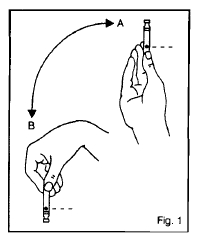
Figure 1
Assemble your insulin delivery device following the directions in your instruction manual.
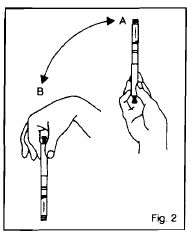
Figure 2
For subsequent injections when a cartridge is already in the device, turn the device up and down between positions A and B - See Figure 2. Do this at least 10 times until the liquid appears uniformly white and cloudy. Follow the directions in your insulin delivery device instruction manual.
Note: Never initiate a new injection unless there is sufficient insulin in the cartridge to ensure proper mixing (the glass ball needs adequate room for movement to mix the suspension).
PenFill cartridges may contain a small amount of air bubbles. To prevent an injection of air and to make certain a full dose of insulin is injected, an air shot must be done before each injection. Directions for performing an air shot are provided in your delivery device instruction manual.
GIVING THE INJECTION
1. The following areas are suitable for subcutaneous insulin injection: thighs, upper arms, buttocks, abdomen. Do not change areas without consulting your physician. The actual point of injection should be changed each time; injection sites should be about an inch apart.
2. The injection site should be clean and dry. Pinch up skin area to be injected and hold it firmly.
3. Hold the device like a pencil and push the needle quickly and firmly into the pinched-up area.
4. Release the skin and push the push button all the way in to inject insulin beneath the skin. After the injection, the needle should remain under the skin for at least 6 seconds. Keep the push button fully depressed until the needle is withdrawn from the skin. This will ensure that the full dose has been injected.
5. Do not inject into a muscle unless your physician has advised it. You should never inject insulin into a vein. Follow the directions for use of your insulin delivery device.
6. Remove the needle. If slight bleeding occurs, press lightly with a dry cotton swab for a few seconds - do not rub.
Note: Use the injection technique recommended by your physician.
USAGE IN PREGNANCY
It is particularly important to maintain good control of your diabetes during pregnancy and special attention must be paid to your diet, exercise and insulin regimens. If you are pregnant or nursing a baby, consult your physician or nurse educator.
INSULIN REACTION AND SHOCK
Insulin reaction (hypoglycemia) occurs when the blood glucose falls very low. This can happen if you take too much insulin, miss or delay a meal, exercise more than usual, or work too hard without eating, or become ill (especially with vomiting or fever). Hypoglycemia can also happen if you combine insulin therapy and other medications that lower blood glucose, such as oral antidiabetic agents or other prescription and over-the-counter drugs. The first symptoms of an insulin reaction usually come on suddenly. They may include a cold sweat, fatigue, nervousness or shakiness, rapid heartbeat, or nausea. Personality change or confusion may also occur. If you drink or eat something right away (a glass of milk or orange juice, or several sugar candies), you can often stop the progression of symptoms. If symptoms persist, call your physician - an insulin reaction can lead to unconsciousness. If a reaction results in loss of consciousness, emergency medical care should be obtained immediately. If you have had repeated reactions or if an insulin reaction has led to a loss of consciousness, contact your physician. Severe hypoglycemia can result in temporary or permanent impairment of brain function and death.
In certain cases, the nature and intensity of the warning symptoms of hypoglycemia may change. A few patients have reported that after being transferred to human insulin, the early warning symptoms of hypoglycemia were less pronounced than they had been with animal-source insulin.
DIABETIC KETOACIDOSIS AND COMA
Diabetic ketoacidosis may develop if your body has too little insulin. The most common causes are acute illness or infection or failure to take enough insulin by injection. If you are ill, you should check your urine for ketones. The symptoms of diabetic ketoacidosis usually come on gradually, over a period of hours or days, and include a drowsy feeling, flushed face, thirst and loss of appetite. Notify your physician right away if the urine test is positive for ketones (acetone) or if you have any of these symptoms. Fast, heavy breathing and rapid pulse are more severe symptoms and you should have medical attention right away. Severe, sustained hyperglycemia may result in diabetic coma and death.
ADVERSE REACTIONS
A few people with diabetes develop red, swollen and itchy skin where the insulin has been injected. This is called a “local reaction” and it may occur if the injection is not properly made, if the skin is sensitive to the cleansing solution, or if you are allergic to the insulin being used.
If you have a local reaction, tell your physician.
Generalized insulin allergy occurs rarely, but when it does it may cause a serious reaction, including skin rash over the body, shortness of breath, fast pulse, sweating, and a drop in blood pressure. If any of these symptoms develop, you should seek emergency medical care.
If severe allergic reactions to insulin have occurred (i.e., generalized rash, swelling or breathing difficulties) you should be skin-tested with each new insulin preparation before it is used.
IMPORTANT NOTES
1. A change in the type, strength, species or purity of insulin could require a dosage adjustment. Any change in insulin should be made under medical supervision.
2. To avoid possible transmission of disease, PenFill cartridge should not be shared.
3. Before use, check that the PenFill cartridge is intact (e.g. no cracks). Do not use if any damage is visible, or if the part of the rubber piston that you see is wider than the white bar code band.
4. You may have learned how to test your urine or your blood for glucose. It is important to do these tests regularly and to record the results for review with your physician or nurse educator.
5. If you have an acute illness, especially with vomiting or fever, continue taking your insulin. If possible, stay on your regular diet. If you have trouble eating, drink fruit juices, regular soft drinks, or clear soups; if you can, eat small amounts of bland foods. Test your urine for glucose and ketones and, if possible, test your blood glucose. Note the results and contact your physician for possible insulin dose adjustment. If you have severe and prolonged vomiting, seek emergency medical care.
6. You should always carry identification which states that you have diabetes.
7. Always ask your physician or pharmacist before taking any drug.
8. Do not try to refill a PenFill cartridge.
Always consult your physician if you have any questions about your condition or the use of insulin.
Helpful information for people with diabetes is published by the American Diabetes Association, 1660 Duke Street, Alexandria, VA 22314
Date of issue: November 18, 2005
Protected by U.S. Patent No. 6,126,646 and No. 5,693,027 and Des. 347,894 and other U.S. Patents Pending, recommended for use with Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery devices, with or without the addition of a NovoPen 3 PenMate, and Novo Nordisk pen needles.
© 2004/2005 Novo Nordisk Inc.
Novo Nordisk®, Novolin®, PenFill®, NovoPen®, PenMate®, NovoFine® and Lente® are registered trademarks owned by Novo Nordisk A/S.
Novo Nordisk Inc.
Princeton, NJ 08540
Call 1-800-727-6500 for additional information
www.novonordisk-us.com
Manufactured by
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
Information for the Patient
NOVOLIN® N INNOLET®
NPH, Human Insulin Isophane Suspension (recombinant DNA origin) is a 3 mL disposable prefilled insulin syringe
100 units/mL (U-100)
Please read both sides of this insert carefully before using this product.
Novolin® N InnoLet® is for single-person use only.
See Important notes section.
WARNING
ANY CHANGE OF INSULIN SHOULD BE MADE CAUTIOUSLY AND ONLY UNDER MEDICAL SUPERVISION. CHANGES IN PURITY, STRENGTH, BRAND (MANUFACTURER), TYPE (REGULAR, NPH, LENTE®, ETC.), SPECIES (BEEF, PORK, BEEF-PORK, HUMAN), AND/OR METHOD OF MANUFACTURE (RECOMBINANT DNA VERSUS ANIMAL-SOURCE INSULIN) MAY RESULT IN THE NEED FOR A CHANGE IN DOSAGE.
SPECIAL CARE SHOULD BE TAKEN WHEN THE TRANSFER IS FROM A STANDARD BEEF OR MIXED SPECIES INSULIN TO A PURIFIED PORK OR HUMAN INSULIN. IF A DOSAGE ADJUSTMENT IS NEEDED, IT WILL USUALLY BECOME APPARENT EITHER IN THE FIRST FEW DAYS OR OVER A PERIOD OF SEVERAL WEEKS. ANY CHANGE IN TREATMENT SHOULD BE CAREFULLY MONITORED.
PLEASE READ THE SECTIONS “INSULIN REACTION AND SHOCK” AND “DIABETIC KETOACIDOSIS AND COMA” FOR SYMPTOMS OF HYPOGLYCEMIA (LOW BLOOD GLUCOSE) AND HYPERGLYCEMIA (HIGH BLOOD GLUCOSE).
INSULIN USE IN DIABETES
Your physician has explained that you have diabetes and that your treatment involves injections of insulin or insulin therapy combined with an oral antidiabetic medicine. Insulin is normally produced by the pancreas, a gland that lies behind the stomach. Without insulin, glucose (a simple sugar made from digested food) is trapped in the bloodstream and cannot enter the cells of the body. Some patients who don’t make enough of their own insulin, or who cannot use the insulin they do make properly, must take insulin by injection in order to control their blood glucose levels. Each case of diabetes is different and requires direct and continued medical supervision.
Your physician has told you the type, strength and amount of insulin you should use and the time(s) at which you should inject it, and has also discussed with you a diet and exercise schedule. You should contact your physician if you experience any difficulties or if you have questions.
TYPES OF INSULINS
Standard and purified animal insulins as well as human insulins are available. Standard and purified insulins differ in their degree of purification and content of noninsulin material. Standard and purified insulins also vary in species source; they may be of beef, pork, or mixed beef and pork origin. Human insulin is identical in structure to the insulin produced by the human pancreas, and thus differs from animal insulins. Insulins vary in time of action; see Product description for additional information.
Your physician has prescribed the insulin that is right for you; be sure you have purchased the correct insulin and check it carefully before you use it.
PRODUCT DESCRIPTION
This package contains Novolin® N in an InnoLet® disposable prefilled insulin syringe. Novolin® N is commonly known as NPH, Human Insulin Isophane Suspension (recombinant DNA origin). The concentration of this product is 100 units of insulin per milliliter. It is a cloudy or milky suspension of human insulin with protamine and zinc. The insulin substance (the cloudy material) settles at the bottom of the insulin reservoir, therefore, the Novolin® N InnoLet® must be rotated up and down so that the contents are uniformly mixed before a dose is given. Novolin® N has an intermediate duration of action. The effect of Novolin® N begins approximately 1½ hours after injection. The effect is maximal between 4 and 12 hours. The full duration of action may last up to 24 hours after injection.
The time course of action of any insulin may vary considerably in different individuals, or at different times in the same individual. Because of this variation, the time periods listed here should be considered as general guidance only.
This human insulin (recombinant DNA origin) is structurally identical to the insulin produced by the human pancreas. This human insulin is produced by recombinant DNA technology utilizing Saccharomyces cerevisiae (bakers’ yeast) as the production organism.
STORAGE
Novolin® N InnoLet® should be stored in a cold (36° - 46°F [2° - 8°C]) place, preferably in a refrigerator, but not in the freezing compartment. Do not let it freeze. Keep Novolin® N InnoLet ® in the carton so that it will stay clean and protected from light. The Novolin® N InnoLet® that you are currently using should not be refrigerated but should be kept as cool as possible (below 86°F [30° C]) and away from direct heat and light. Unrefrigerated Novolin® N InnoLet® must be discarded after 14 days, even if it still contains Novolin N.
Never use Novolin® N InnoLet® after the expiration date printed on the label and carton.
Never use any Novolin® N InnoLet® if the precipitate (the white deposit) has become lumpy or granular in appearance or has formed a deposit of solid particles on the wall of the insulin reservoir. This insulin should not be used if the liquid in the insulin reservoir remains clear after it has been mixed.
IMPORTANT
Failure to comply with the following antiseptic measures may lead to infections at the injection site.
- Disposable needles are for single use; they should be used only once and destroyed.
- Clean your hands and the injection site with soap and water or with alcohol.
- Wipe the rubber stopper on the insulin cartridge with an alcohol swab.
PREPARING THE INJECTION
Never place a single-use disposable needle on your Novolin® N InnoLet® until you are ready to give an injection, and remove the needle immediately after each injection. Follow the directions for use of the Novolin® N InnoLet® on the reverse side of this insert.
Novolin® N InnoLet® may contain a small amount of air. To prevent an injection of air and make certain insulin is delivered, an air shot must be done before each injection. Directions for performing an air shot are provided on the reverse side of this insert.
GIVING THE INJECTION
1. The following areas are suitable for subcutaneous insulin injection: thighs, upper arms, buttocks, abdomen. Do not change areas without consulting your physician. The actual point of injection should be changed each time; injection sites should be about an inch apart.
2. The injection site should be clean and dry. Pinch up skin area to be injected and hold it firmly.
3. Hold the device upright and push the needle quickly and firmly into the pinched-up area. Release the skin and push the push-button all the way in to inject insulin beneath the skin. After the injection, the needle should remain under the skin for at least 6 seconds. Keep the push-button fully depressed until the needle is withdrawn from the skin. This will ensure that the full dose has been delivered.
4. Do not inject into a muscle unless your physician has advised it. You should never inject insulin into a vein.
5. Remove the needle. If slight bleeding occurs, press lightly with a dry cotton swab for a few seconds - do not rub.
For additional information see Giving the injection on the reverse side of this insert.
USAGE IN PREGNANCY
It is particularly important to maintain good control of your diabetes during pregnancy and special attention must be paid to your diet, exercise and insulin regimens. If you are pregnant or nursing a baby, consult your physician or nurse educator.
INSULIN REACTION AND SHOCK
Insulin reaction (hypoglycemia) occurs when the blood glucose falls very low. This can happen if you take too much insulin, miss or delay a meal, exercise more than usual or work too hard without eating, or become ill (especially with vomiting or fever).
Hypoglycemia can also happen if you combine insulin therapy and other medications that lower blood glucose, such as oral antidiabetic agents or other prescription and over-the-counter drugs. The first symptoms of an insulin reaction usually come on suddenly. They may include a cold sweat, fatigue, nervousness or shakiness, rapid heartbeat, or nausea. Personality change or confusion may also occur. If you drink or eat something right away (a glass of milk or orange juice, or several sugar candies), you can often stop the progression of symptoms. If symptoms persist, call your physician - an insulin reaction can lead to unconsciousness. If a reaction results in loss of consciousness, emergency medical care should be obtained immediately. If you have had repeated reactions or if an insulin reaction has led to a loss of consciousness, contact your physician.
Severe hypoglycemia can result in temporary or permanent impairment of brain function and death.
In certain cases, the nature and intensity of the warning symptoms of hypoglycemia may change. A few patients have reported that after being transferred to human insulin, the early warning symptoms of hypoglycemia were less pronounced than they had been with animal-source insulin.
DIABETIC KETOACIDOSIS AND COMA
Diabetic ketoacidosis may develop if your body has too little insulin. The most common causes are acute illness or infection or failure to take enough insulin by injection. If you are ill you should check your urine for ketones. The symptoms of diabetic ketoacidosis usually come on gradually, over a period of hours or days, and include a drowsy feeling, flushed face, thirst and loss of appetite. Notify your physician right away if the urine test is positive for ketones (acetone) or if you have any of these symptoms. Fast, heavy breathing and rapid pulse are more severe symptoms and you should have medical attention right away. Severe, sustained hyperglycemia may result in diabetic coma and death.
ADVERSE REACTIONS
A few people with diabetes develop red, swollen and itchy skin where the insulin has been injected. This is called a “local reaction” and it may occur if the injection is not properly made, if the skin is sensitive to the cleansing solution, or if you are allergic to the insulin being used. If you have a local reaction, tell your physician.
Generalized insulin allergy occurs rarely, but when it does it may cause a serious reaction, including skin rash over the body, shortness of breath, fast pulse, sweating, and a drop in blood pressure. If any of these symptoms develop, you should seek emergency medical care.
If severe allergic reactions to insulin have occurred (i.e., generalized rash, swelling or breathing difficulties) you should be skin-tested with each new insulin preparation before it is used.
IMPORTANT NOTES
1. A change in the type, strength, species or purity of insulin could require a dosage adjustment. Any change in insulin should be made under medical supervision.
2. To avoid possible transmission of disease, Novolin® N InnoLet® is for single-person use only.
3. You may have learned how to test your urine or your blood for glucose. It is important to do these tests regularly and to record the results for review with your physician or nurse educator.
4. If you have an acute illness, especially with vomiting or fever, continue taking your insulin. If possible, stay on your regular diet. If you have trouble eating, drink fruit juices, regular soft drinks, or clear soups; if you can, eat small amounts of bland foods. Test your urine for glucose and ketones and, if possible, test your blood glucose. Note the results and contact your physician for possible insulin dose adjustment. If you have severe and prolonged vomiting, seek emergency medical care.
5. You should always carry identification which states that you have diabetes.
6. Always ask your physician or pharmacist before taking any drug.
Always consult your physician if you have any questions about your condition or the use of insulin.
Helpful information for people with diabetes is published by American Diabetes Association, 1701 N. Beauregard Street, Alexandria, VA 22311
Date of issue: June 2008
© 2002/2008 Novo Nordisk A/S
Call 800-727-6500 for additional information.
Novo Nordisk Inc.
Princeton, NJ 08540
www.novonordisk-us.com
Manufactured by
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
Novo Nordisk®, Novolin®, Lente®, NovoFine® and InnoLet® are trademarks owned by Novo Nordisk A/S
Novolin® N InnoLet®
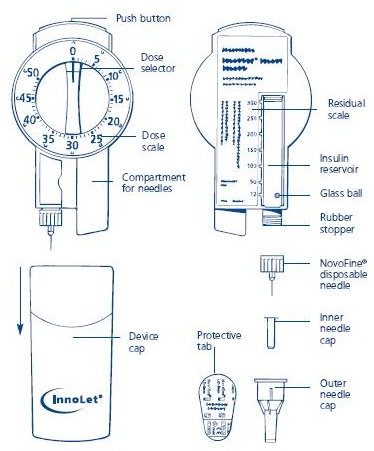
NOVOLIN® N INNOLET® DIRECTIONS FOR USE
Novolin® N InnoLet® is a disposable dial-a-dose insulin delivery system able to deliver 1-50 units in increments of 1 unit. Novolin® N InnoLet® is designed and recommended for use with NovoFine® single-use needles.
Novolin® N InnoLet® is not recommended for the blind or severely visually impaired patients without the assistance of a sighted individual trained in the proper use of this product.
Please read these instructions completely before using this device.
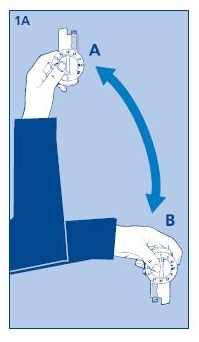
1. Preparing the Novolin® N InnoLet®:
Pull off the device cap.
1A. Turn the Novolin® N InnoLet® up and down between positions A and B so the glass ball is moved from one end of the insulin reservoir to the other. Do this at least 10 times, until the liquid appears uniformly white and cloudy.
To ensure even mixing of the remaining insulin there must be at least 12 units of insulin left in the reservoir. If there are less than 12 units left, do not use the Novolin® N InnoLet®.
Wipe rubber stopper with an alcohol swab.
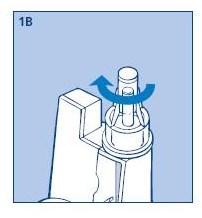
1B. Remove the protective tab from the disposable needle and screw the needle onto the Novolin® N InnoLet®. Never place a disposable needle on your Novolin® N InnoLet® until you are ready to give an injection. Remove the needle immediately after use. If the needle is not removed, some liquid may be expelled from the Novolin® N InnoLet® causing a change in insulin concentration (strength).
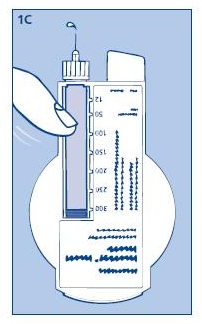
1C. Giving the air shot prior to each injection:
Small amounts of air may collect in the needle and insulin reservoir during normal use. To avoid the injection of air and ensure proper dosing, dial 2 units by turning the dose selector clockwise. Hold the Novolin® N InnoLet® with the needle up and tap the Novolin® N InnoLet® gently with your finger so any air bubbles collect in the top of the reservoir. Remove both the plastic outer and inner needle caps.
With the needle pointing up, press the push button as far as it will go and the dose selector returns to zero. See if a drop of insulin appears at the needle tip (see fig. 1C). If not, repeat the procedure until insulin appears.
Before the first use of Novolin® N InnoLet® you may need to perform up to 6 air shots to get a drop of insulin at the needle tip. If you need to make more than 6 air shots, do not use, and return the product to Novo Nordisk. A small air bubble may remain but it will not be injected because the operating mechanism prevents the reservoir from being completely emptied.
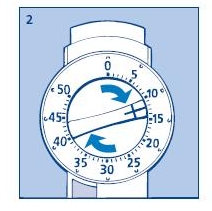
2. Setting the dose
Always check that the push button is fully depressed and the dose selector is set to zero. Hold the Novolin® N InnoLet® in front of you and dial the dose selector clockwise to set the required dose. Do not put your hand over the push button when dialing the dose. If the button is not allowed to rise freely, insulin will be pushed out of the needle. When setting your dose, you will hear a click for every single unit dialed. Do not rely on this clicking sound as a means of determining your dose. If you have set a wrong dose, simply dial the dose selector forward or backwards until the right number of units has been set.
50 units is the maximum dose.
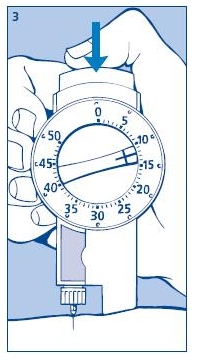
3. Giving the injection
Use the injection technique recommended by your doctor. Check that you have set the proper dose and depress the push button as far as it will go. Make sure not to block the dose selector while injecting as the dose selector must be allowed to return to zero when you press the push button. When depressing the push button you may hear a clicking sound. Do not rely on this clicking sound as a means of confirming delivery of your dose.
After making the injection, unscrew the needle and discard appropriately. Replace the device cap.
Health care professionals, relatives, and other care-givers should follow general precautionary measures for removal and disposal of needles to eliminate the risk of unintended needle penetration.
For additional information see Giving the injection on the reverse side of this insert.
Subsequent injections
Always check that the push button is fully depressed before using the Novolin® N InnoLet® again. If not, turn the dose selector until the push button is completely down. Then proceed as stated under steps 1-3. The numbers on the insulin reservoir can be used to estimate the amount of insulin left in the Novolin® N InnoLet®. These numbers are not used for measuring the insulin dose.
You cannot set a dose greater than the number of units remaining in the reservoir.
4. Function check
If you think that your Novolin® N InnoLet® is not working properly, follow this procedure:
a. Screw on a new NovoFine needle.
b. Perform air shot as described in section 1C.
c. Put the outer needle cap onto the needle.
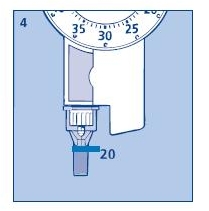
d. Dispense 20 units into the needle cap.
The insulin will fill the lower part of the cap (as shown in fig. 4).
If the Novolin® N InnoLet® has released too much or too little insulin, repeat the test. If it happens again, contact Novo Nordisk and do not use your Novolin® N InnoLet®.
5. Important notes
a. If you need to perform more than 6 air shots before the first use of Novolin® N InnoLet® to get a drop of insulin at the needle tip, do not use.
b. Remember to perform an air shot before each injection (see fig. 1C).
c. Care should be taken not to drop the Novolin® N InnoLet® or subject it to impact.
d. Remember to keep the Novolin® N InnoLet® that you are currently using with you; don’t leave it in a car or other location where extremes of temperature can occur.
e. Novolin® N InnoLet® is designed and recommended for use with NovoFine disposable needles.
f. Never place a disposable needle on the Novolin® N InnoLet® until you are ready to use it. Remove the needle immediately after use.
g. Discard the used Novolin® N InnoLet® carefully, without the needle attached.
h. Always carry a spare Novolin® N InnoLet® with you in case your Novolin® N InnoLet® is damaged or lost.
i. Novo Nordisk cannot be held responsible for adverse reactions occurring as a consequence of using the insulin delivery system with products that are not recommended by Novo Nordisk.
j. Keep Novolin® N InnoLet® out of the reach of children.
© 2002/2008 Novo Nordisk A/S
Call 800-727-6500 for additional information.
Novo Nordisk Inc.
Princeton, NJ 08540
www.novonordisk-us.com
Manufactured by
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
Novo Nordisk®, Novolin®, Lente®, NovoFine® and InnoLet® are trademarks owned by Novo Nordisk A/S
U.S. Patents Nos. 5,947,934, 6,074,372, 6,110,149, 6,302,869, 6,524,280, 6,379,339, 6,582,404, and other U.S. patents pending.
Principal Display Panel - 10mL Vial
NDC 0169-1834-11
Novolin® N
NPH, Human Insulin Isophane Suspension
(recombinant DNA origin)
100 units/ml
10 ml
Novo Nordisk
Principal Display Panel - 3mL PenFill
NDC 0169-3474-18
Novolin® N PenFill 3 mL
NPH, Human Insulin
Isophane Suspension
(recombinant DNA origin)
List 347418
For use with Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery devices
Keep in a cold place
Avoid freezing
Shake carefully to mix before use
List 347418
100 uits/mL (U-100)
5x3 mL
cartridge
novo nordisk
Principal Display Panel - 3mL InnoLet
NDC 0169-2314-21 List 231421
Novolin® N InnoLet®
NPH, Human Insulin Isophane Suspension
(recombinant DNA origin)
100 units/mL (U-100)
5x3 mL Innolet® Prefilled Insulin Syringes
Keep in a cold place between 36° - 46°F (2° - 8°C)
Avoid freezing
novo nordisk
| NOVOLIN
N
human insulin injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019959 | 07/01/1991 | |
| NOVOLIN
N
human insulin injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019959 | 07/01/1991 | |
| NOVOLIN
N
human insulin injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019959 | 07/01/1991 | |
| Labeler - Novo Nordisk (622920320) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Novo Nordisk Pharmaceuticals Industries Inc. | 622920320 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Novo Nordisdk A/S | 312296002 | MANUFACTURE | |