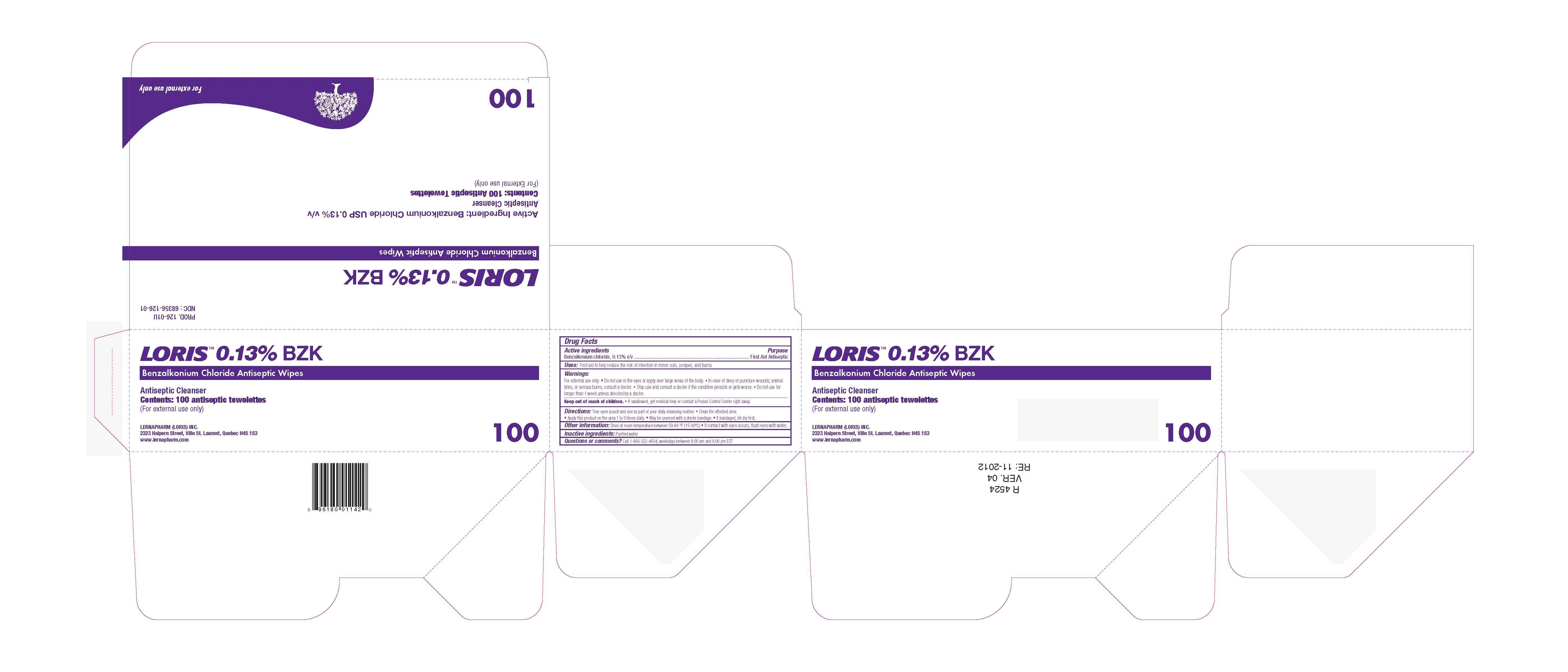
LORIS BZK ANTISEPTIC WIPES- benzalkonium chloride swab
LernaPharm Loris Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For External Use Only
Directions
- Tear open pouch and use as part of your daily cleansing routine.
- Clean the affected area.
- Apply this product on the area 1 to 3 times daily.
- May be covered with a sterile bandage.
- If bandaged, let dry first.
| LORIS BZK
ANTISEPTIC WIPES
benzalkonium chloride swab |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - LernaPharm Loris Inc. (206940905) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LernaPharm Loris Inc. | 206940905 | manufacture(68356-126), label(68356-126) | |
Revised: 04/2013
Document Id: 15131d4b-3cba-4717-99a6-8bd409a78f09
Set id: a448de7b-4d96-4d53-ae1a-0bd38a2fb4a2
Version: 6
Effective Time: 20130416
LernaPharm Loris Inc.