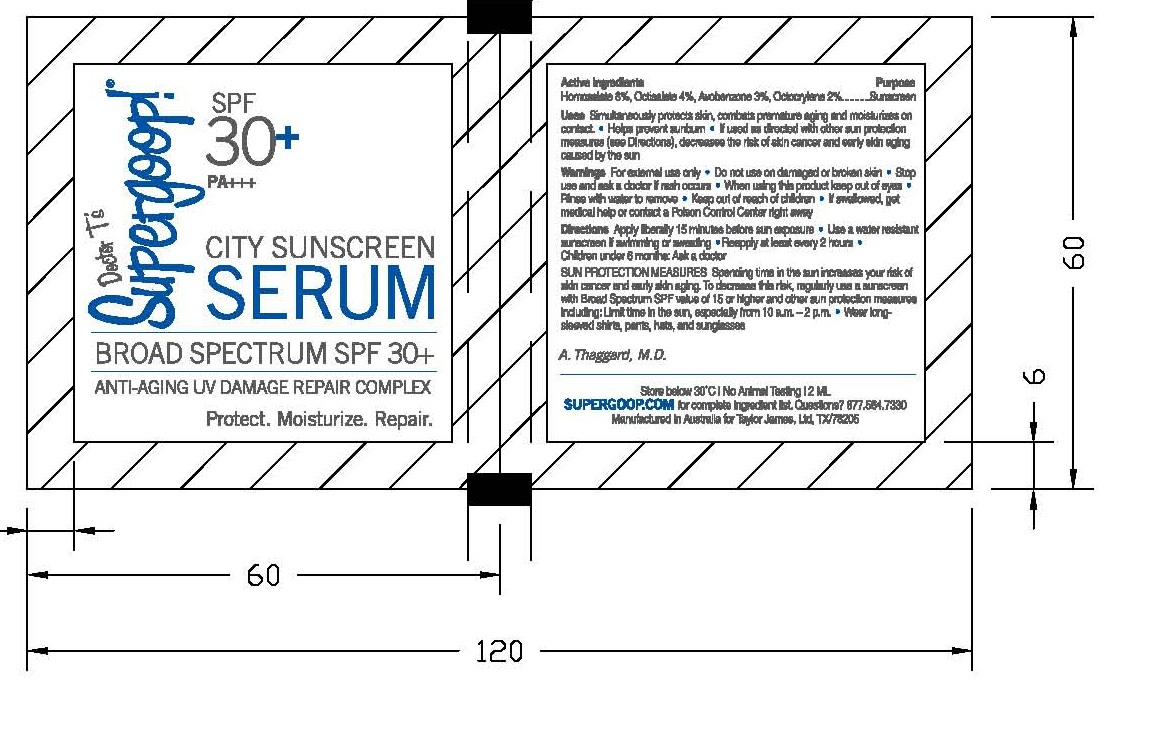
CITY SUNSCREEN SERUM SPF 30 SUPERGOOP- homosalate, octisalate, avobenzone and octocrylene cream
Taylor James, Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
City Sunscreen Serum
Active ingredients: Purpose
Homosalate 8%, Octisalate 4%, Avobenzene 3%, Octocrylene 2% Sunscreen
Uses Simultaneously protects skin, combats premature aging and moisturizes on contact
helps prevent sunburn
If used as directed with other sun protection measures (see directions), decreases the risk of skin cancer and early skin aging skin aging caused by the sun
For external use only.
Do not use on damaged or broken skin
When using this product, keep out of of eyes.
Rinse with water to remove.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions: Apply liberally 15 minutes before sun exposure
Use a water resistant sunscreen if swimming or sweating
Reapply at least every 2 hours
children under 6 months: ask a doctor
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreenwith Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun,especially from 10 a.m. -2p.m.
Wear long-sleeved shirts, pants, hats, and sunglasses
Inactive Ingredients Purified water (Aqua), Cyclometicone, Isostearyl Neopentanoate, Glycerin, Ceteareth-20, Polypropylene, Cetearyl Alcohol, Xanthan Gum, d-Panthenol, Octanohydroxamic acid, Caprylyl Glycol, Silica, Triacontanyl PVP, Cetyl Dimethicone, Ammonium Acryloyldimethyltaurate/VP Copolymer, PEG-40 Stearate, Tocopheryl, Disodium EDTA, Pentylene Glycol, Pantheyl Triacetate, Sodium Lactate, Lactic Acid, Serine, Urea, Sorbitol, Sodium Chloride, Allantoin, Oleyl Alcohol, Ethyl Linoleate.
| CITY SUNSCREEN SERUM SPF 30
SUPERGOOP
homosalate, octisalate, avobenzene, octocrylene cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Taylor James, Ltd. (033381850) |
| Registrant - Baxter Laboratories Pty. Ltd. (740537709) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Laboratories Pty. Ltd. | 740537709 | manufacture(75936-111) | |