NAPROXEN SODIUM
-
naproxen sodium tablet, coated
Goldline Laboratories, Inc.
----------
NAPROXEN SODIUMTABLETS USP, 220 mg
PAIN RELIEVER / FEVER REDUCER (NSAID)
Drug Facts
Active ingredient (in each tablet)
Naproxen sodium 220 mg
(naproxen 200 mg) (NSAID)1
- 1
- nonsteroidal anti-inflammatory drug
Purposes
Pain reliever/fever reducer
Uses
- temporarily relieves minor aches and pains due to:
- minor pain of arthritis
- backache
- headache
- the common cold
- muscular aches
- menstrual cramps
- toothache
- temporarily reduces fever
Warnings
Allergy alert: Naproxen sodium may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- asthma (wheezing)
- skin reddening
- facial swelling
- shock
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause stomach bleeding.
The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing an NSAID (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if you have
- problems or serious side effects from taking pain relievers or fever reducers
- stomach problems that last or come back, such as heartburn, upset stomach, or stomach pain
- ulcers
- asthma
- high blood pressure
- taken a diuretic
- bleeding problems
- heart or kidney disease
- reached age 60 or older
Ask a doctor or pharmacist before use if you are
- taking any other drug containing an NSAID (prescription or nonprescription)
- taking a blood thinning (anticoagulant) or steroid drug
- under a doctor's care for any serious condition
- taking any other drug
When using this product
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
Stop use and ask a doctor if
- you feel faint, vomit blood, or have bloody or black stools. These are signs of stomach bleeding.
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- you have difficulty swallowing
- it feels like the pill is stuck in your throat
- you develop heartburn
- stomach pain or upset gets worse or lasts
- redness or swelling is present in the painful area
- any new symptoms appear
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use naproxen sodium during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless directed by a doctor (see Warnings)
- drink a full glass of water with each dose
| Adults and children 12 years and older |
|
| Children under 12 years |
|
Other information
- each tablet contains: sodium 20 mg
- store at 20-25°C (68-77°F). Avoid high humidity and excessive heat above 40°C (104°F).
Inactive ingredients
FD&C blue no. 2 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, talc, titanium dioxide
Questions?
call 1-888-838-2872, weekdays, 8 AM - 5 PM Eastern Time
**This product is not manufactured or distributed by Bayer HealthCare LLC, owner of the registered trademark Aleve®.
Dist. by: GOLDLINE LABORATORIES, INC.
Sellersville, PA 18960
Dist. 1996 0409REV 21
PRINCIPAL DISPLAY PANEL - 24 Tablets Bottle
Goldline
TAMPER-EVIDENT
NDC 0182-1097-16
NAPROXEN
SODIUM
TABLETS USP, 220 mg
PAIN RELIEVER / FEVER REDUCER (NSAID)
STRENGTH TO LAST 12 HOURS
24 TABLETS

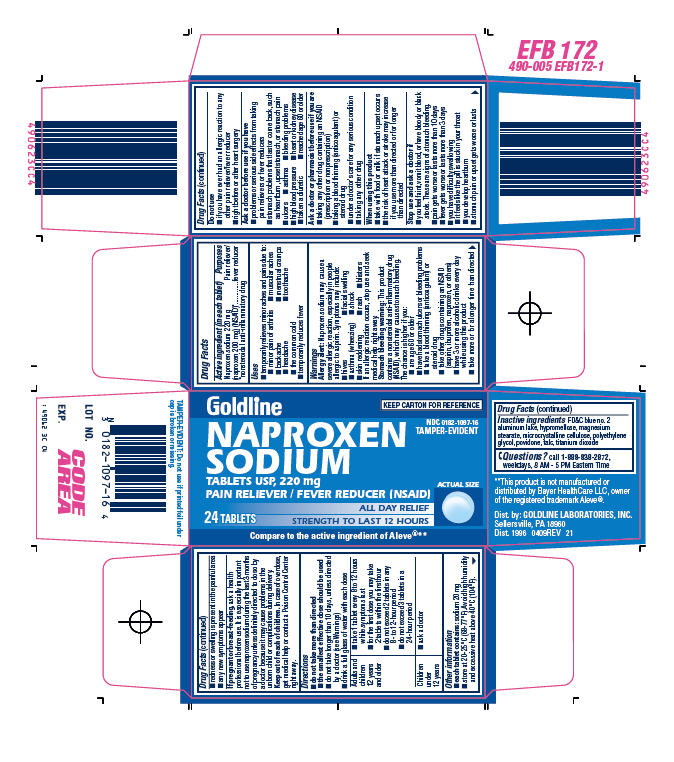
PRINCIPAL DISPLAY PANEL - 24 Tablets Carton
Goldline
KEEP CARTON FOR REFERENCE
NDC 0182-1097-16
TAMPER-EVIDENT
NAPROXEN
SODIUM
TABLETS USP, 220 mg
PAIN RELIEVER / FEVER REDUCER (NSAID)
ALL DAY RELIEF
STRENGTH TO LAST 12 HOURS
24 TABLETS
ACTUAL SIZE
Compare to the active ingredient of Aleve®**
PRINCIPAL DISPLAY PANEL - 50 Tablets Bottle
Goldline
TAMPER-EVIDENT
NDC 0182-1097-19
NAPROXEN
SODIUM
TABLETS USP, 220 mg
PAIN RELIEVER / FEVER REDUCER (NSAID)
STRENGTH TO LAST 12 HOURS
50 TABLETS

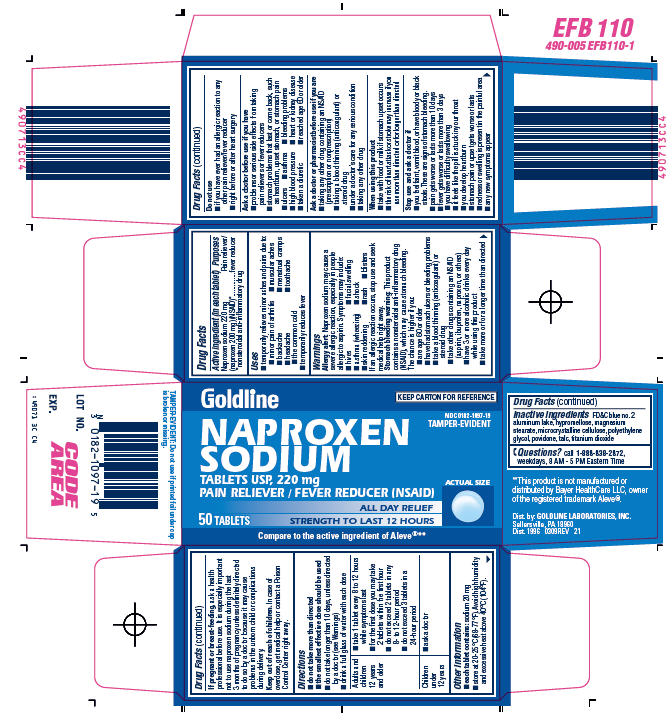
PRINCIPAL DISPLAY PANEL - 50 Tablets Carton
Goldline
KEEP CARTON FOR REFERENCE
NDC 0182-1097-19
TAMPER-EVIDENT
NAPROXEN
SODIUM
TABLETS USP, 220 mg
PAIN RELIEVER / FEVER REDUCER (NSAID)
ALL DAY RELIEF
STRENGTH TO LAST 12 HOURS
50 TABLETS
ACTUAL SIZE
Compare to the active ingredient of Aleve®**
| NAPROXEN SODIUM
naproxen sodium tablet, coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA074661 | 05/15/2009 | |
| Labeler - Goldline Laboratories, Inc. (032349292) |