LORABID - loracarbef capsule
Physicians Total Care, Inc.
----------
DESCRIPTION
Lorabid® (loracarbef, USP) is a synthetic β-lactam antibiotic of the carbacephem class for oral administration. Chemically, carbacephems differ from cephalosporin-class antibiotics in the dihydrothiazine ring where a methylene group has been substituted for a sulfur atom.
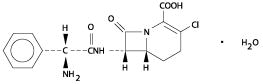
The chemical name for loracarbef is: (6R, 7S)-7-[(R)-2-amino-2-phenylacetamido]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, monohydrate. It is a white to off-white solid with a molecular weight of 367.8. The empirical formula is C16H16ClN3O4•H2O. The structural formula is:
Lorabid Pulvules® (loracarbef capsules, USP) and Lorabid for Oral Suspension (loracarbef for oral suspension, USP) are intended for oral administration only.
Each Pulvule contains loracarbef equivalent to 200 mg (0.57 mmol) or 400 mg (1.14 mmol) anhydrous loracarbef activity. They also contain cornstarch, dimethicone, FD & C Blue No. 2, gelatin, iron oxides, magnesium stearate, and titanium dioxide.
After reconstitution, each 5 mL of Lorabid for Oral Suspension contains loracarbef equivalent to 100 mg (0.286 mmol) or 200 mg (0.57mmol) anhydrous loracarbef activity. The suspensions also contain cellulose, F D & C Red No. 40, flavors, methylparaben, propylparaben, simethicone emulsion, sodium carboxymethylcellulose, sucrose, and xanthan gum.
CLINICAL PHARMACOLOGY
Loracarbef, after oral administration, was approximately 90% absorbed from the gastrointestinal tract. When capsules were taken with food, peak plasma concentrations were 50% to 60% of those achieved when the drug was administered to fasting subjects and occurred from 30 to 60 minutes later. Total absorption, as measured by urinary recovery and area under the plasma concentration versus time curve (AUC), was unchanged. The effect of food on the rate and extent of absorption of the suspension formulation has not been studied to date.
The pharmacokinetics of loracarbef were linear over the recommended dosage range of 200 to 400 mg, with no accumulation of the drug noted when it was given twice daily.
Average peak plasma concentrations after administration of 200-mg or 400-mg single doses of loracarbef as capsules to fasting subjects were approximately 8 and 14 μg/mL, respectively, and were obtained within 1.2 hours after dosing. The average peak plasma concentration in adults following a 400-mg single dose of suspension was 17 μg/mL and was obtained within 0.8 hour after dosing (see Table).
| Mean Plasma Loracarbef | ||
| Concentrations (μg/mL) | ||
| Dosage | Peak | Time to Peak |
| (mg) | Cmax | Tmax |
| Capsule (single dose) | ||
| 200 mg | 8 | 1.2 h |
| 400 mg | 14 | 1.2 h |
| Suspension (single dose) | ||
| 400 mg (adult) | 17 | 0.8 h |
| 7.5 mg/kg (pediatric) | 13 | 0.8 h |
| 15 mg/kg (pediatric) | 19 | 0.8 h |
Following administration of 7.5 and 15 mg/kg single doses of oral suspension to children, average peak plasma concentrations were 13 and 19 μg/mL, respectively, and were obtained within 40 to 60 minutes.
This increased rate of absorption (suspension > capsule) should be taken into consideration if the oral suspension is to be substituted for the capsule, and capsules should not be substituted for the oral suspension in the treatment of otitis media (see DOSAGE AND ADMINISTRATION).
The elimination half-life was an average of 1.0 h in patients with normal renal function. Concomitant administration of probenecid decreased the rate of urinary excretion and increased the half-life to 1.5 hours.
In subjects with moderate impairment of renal function (creatinine clearance 10 to 50 mL/min/1.73 m2), following a single 400-mg dose, the plasma half-life was prolonged to approximately 5.6 hours. In subjects with severe renal impairment (creatinine clearance <10 mL/min/1.73 m2), the half-life was increased to approximately 32 hours. During hemodialysis the half-life was approximately 4 hours. In patients with severe renal impairment, the Cmax increased from 15.4 μg/mL to 23 μg/mL (see PRECAUTIONSand DOSAGE AND ADMINISTRATION).
In single-dose studies, plasma half-life and AUC were not significantly altered in healthy elderly subjects with normal renal function.
There is no evidence of metabolism of loracarbef in humans.
Approximately 25% of circulating loracarbef is bound to plasma proteins.
Middle-ear fluid concentrations of loracarbef were approximately 48% of the plasma concentration 2 hours after drug administration in pediatric patients. The peak concentration of loracarbef in blister fluid was approximately half that obtained in plasma. Adequate data on CSF levels of loracarbef are not available.
Microbiology—Loracarbef exerts its bactericidal action by binding to essential target proteins of the bacterial cell wall, leading to inhibition of cell-wall synthesis. It is stable in the presence of some bacterial β-lactamases. Loracarbef has been shown to be active against most strains of the following organisms both in vitro and in clinical infections (see INDICATIONS AND USAGE):
Gram-positive aerobes:
Staphylococcus aureus (including penicillinase-producing strains)
NOTE: Loracarbef (like most β-lactam antimicrobials) is inactive against methicillin-resistant staphylococci.
Staphylococcus saprophyticus
Streptococcus pneumoniae
Streptococcus pyogenes
Gram-negative aerobes:
Escherichia coli
Haemophilus influenzae (including β-lactamase-producing strains)
Moraxella (Branhamella) catarrhalis (including β-lactamase producing strains)
The following in vitro data are available; however, their clinical significance is unknown.
Loracarbef exhibits in vitro minimum inhibitory concentrations (MIC) of 8 μg/mL or less against most strains of the following organisms; however, the safety and efficacy of loracarbef in treating clinical infections due to these organisms have not been established in adequate and well-controlled trials.
Gram-positive aerobes:
Staphylococcus epidermidis
Streptococcus agalactiae (group B streptococci)
Streptococcus bovis
Streptococci, groups C, F, and G viridans group streptococci
Gram-negative aerobes:
Citrobacter diversus
Haemophilus parainfluenzae
Klebsiella pneumoniae
Neisseria gonorrhoeae (including penicillinase-producing strains)
Pasteurella multocida
Proteus mirabilis
Salmonella species
Shigella species
Yersinia enterocolitica
NOTE: Loracarbef is inactive against most strains of Acinetobacter , Enterobacter, Morganella morganii , Proteus vulgaris , Providencia, Pseudomonas , and Serratia.
Anaerobic organisms:
Clostridium perfringens
Fusobacterium necrophorum
Peptococcus niger
Peptostreptococcus intermedius
Propionibacterium acnes
Susceptibility Testing
Diffusion Techniques—Quantitative methods that require measurement of zone diameters give the most precise estimate of the susceptibility of bacteria to antimicrobial agents. One such standardized method1 has been recommended for use with the 30-μg loracarbef disk. Interpretation involves the correlation of the diameter obtained in the disk test with MIC for loracarbef. Reports from the laboratory giving results of the standard single-disk susceptibility test with a 30-μg loracarbef disk should be interpreted according to the following criteria:
| Zone Diameter (mm) | Interpretation |
| ≥18 | (S) Susceptible |
| 15–17 | (MS) Moderately Susceptible |
| ≤14 | (R) Resistant |
A report of “susceptible” implies that the pathogen is likely to be inhibited by generally achievable blood concentrations. A report of “moderately susceptible” indicates that inhibitory concentrations of the antibiotic may be achieved if high dosage is used or if the infection is confined to tissues and fluids (e.g., urine) in which high antibiotic concentrations are attained. A report of “resistant” indicates that achievable concentrations of the antibiotic are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures require the use of laboratory control organisms. The 30-μg loracarbef disk should give the following zone diameters with the NCCLS approved procedure:
| Organism | Zone Diameter (mm) |
| E. coli ATCC 25922 | 23–29 |
| S. aureus ATCC 25923 | 23–31 |
Dilution Techniques—Use a standardized dilution method2 (broth, agar, or microdilution) or equivalent with loracarbef powder. The MIC values obtained should be interpreted according to the following criteria:
| MIC (μg/mL) | Interpretation |
| ≤8 | (S) Susceptible |
| 16 | (MS) Moderately Susceptible |
| ≥32 | (R) Resistant |
As with standard diffusion methods, dilution procedures require the use of laboratory control organisms. Standard loracarbef powder should give the following MIC values with the NCCLS approved procedure:
| Organism | MIC Range (μg/mL) |
| E. coli ATCC 25922 | 0.5–2 |
| S. aureus ATCC 29213 | 0.5–2 |
INDICATIONS AND USAGE
Lorabid is indicated in the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below. (As recommended dosages, durations of therapy, and applicable patient populations vary among these infections, please see DOSAGE AND ADMINISTRATION for specific recommendations.)
Lower Respiratory Tract
Secondary Bacterial Infection of Acute Bronchitis caused by S. pneumoniae , H. influenzae (including β-lactamase-producing strains), or M. catarrhalis (including β-lactamase-producing strains).
Upper Respiratory Tract
Otitis Media † caused by S. pneumonia, H. influenzae (including β-lactamase-producing strains), M. catarrhalis (including β-lactamase-producing strains), or S. pyogenes.
Acute Maxillary Sinusitis † caused by S. pneumoniae, H. influenzae (non-β-lactamase-producing strains only), or M. catarrhalis (including β-lactamase-producing strains). Data are insufficient at this time to establish efficacy in patients with acute maxillary sinusitis caused by β-lactamase-producing strains of H. influenzae.
† NOTE: In a patient population with significant numbers of β-lactamase-producing organisms, loracarbef's clinical cure and bacteriological eradication rates were somewhat less than those observed with a product containing a β-lactamase inhibtor. Lorabid's decreased potential for toxicity compared to products containing β-lactamase inhibitors along with the susceptibility patterns of the common microbes in a given geographic area should be taken into account when considering the use of an antimicrobial (see CLINICAL STUDIES section). For information on use in pediatric patients, seePRECAUTIONS—Pediatric Use.
Pharyngitis and Tonsillitis caused by S. pyogenes. (The usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever, is penicillin administered by the intramuscular route. Lorabid is generally effective in the eradication of S. pyogenes from the nasopharynx; however, data establishing the efficacy of Lorabid in the subsequent prevention of rheumatic fever are not available at present.)
Skin and Skin Structure
Uncomplicated Skin and Skin Structure Infections caused by S. aureus (including penicillinase-producing strains) or S. pyogenes. Abscesses should be surgically drained as clinically indicated.
Urinary Tract
Uncomplicated Urinary Tract Infections (cystitis) caused by E.coli or S. saprophyticus*.
NOTE: In considering the use of Lorabid in the treatment of cystitis, Lorabid's lower bacterial eradication rates and lower potential for toxicity should be weighed against the increased eradication rates and increased potential for toxicity demonstrated by some other classes of approved agents (see CLINICAL STUDIES section).
Uncomplicated Pyelonephritis caused by E. coli.
*Although treatment of infections due to this organism in this organ system demonstrated a clinically acceptable overall outcome, efficacy was studied in fewer than 10 infections.
Culture and susceptibility testing should be performed when appropriate to determine the causative organism and its susceptibility to loracarbef. Therapy may be started while awaiting the results of these studies. Once these results become available, antimicrobial therapy should be adjusted accordingly.
CONTRAINDICATION
Lorabid is contraindicated in patients with known allergy to loracarbef or cephalosporin-class antibiotics.
WARNINGS
BEFORE THERAPY WITH LORABID IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO LORACARBEF, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG β-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO LORABID OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE THE USE OF EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Pseudomembranous colitis has been reported with nearly all antibacterial agents and may range from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with broad-spectrum antibiotics alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of “antibiotic-associated colitis.”
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug effective against C. difficile-associated colitis.
PRECAUTIONS
General—In patients with known or suspected renal impairment (see DOSAGE AND ADMINISTRATION), careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy. The total daily dose of loracarbef should be reduced in these patients because high and/or prolonged plasma antibiotic concentrations can occur in such individuals administered the usual doses. Loracarbef, like cephalosporins, should be given with caution to patients receiving concurrent treatment with potent diuretics because these diuretics are suspected of adversely affecting renal function.
As with other broad-spectrum antimicrobials, prolonged use of loracarbef may result in the overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Loracarbef, as with other broad-spectrum antimicrobials, should be prescribed with caution in individuals with a history of colitis.
Information for Patients—Lorabid should be taken either at least 1 hour prior to eating or at least 2 hours after eating a meal.
Drug Interactions—
Probenecid: As with other β-lactam antibiotics, renal excretion of loracarbef is inhibited by probenecid and resulted in an approximate 80% increase in the AUC for loracarbef (see CLINICAL PHARMACOLOGY).
Carcinogenesis, Mutagenesis, Impairment of Fertility—Although lifetime studies in animals have not been performed to evaluate carcinogenic potential, no mutagenic potential was found for loracarbef in standard tests of genotoxicity, which included bacterial mutation tests and in vitro and in vivo mammalian systems. In rats, fertility and reproductive performance were not affected by loracarbef at doses up to 33 times the maximum human exposure in mg/kg (10 times the exposure based on mg/m2).
Usage in Pregnancy—Pregnancy Category B—Reproduction studies have been performed in mice, rats, and rabbits at doses up to 33 times the maximum human exposure in mg/kg (4, 10, and 4 times the exposure, respectively, based on mg/m2) and have revealed no evidence of impaired fertility or harm to the fetus due to loracarbef. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery—Lorabid has not been studied for use during labor and delivery. Treatment should be given only if clearly needed.
Nursing Mothers—It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Lorabid is administered to a nursing woman.
Pediatric Use—The safety and efficacy of Lorabid have been established for pediatric patients aged six months to twelve years for acute maxillary sinusitis based upon its approval in adults. Use of Lorabid in pediatric patients is supported by pharmacokinetic and safety data in adults and children, and by clinical and microbiologic data from adequate and well-controlled studies of the treatment of acute maxillary sinusitis in adults and of acute otitis media with effusion in children. It is also supported by post-marketing adverse events surveillance. (See CLINICAL PHARMACOLOGY, INDICATIONS AND USAGE, ADVERSE REACTIONS, DOSAGE AND ADMINISTRATION, and CLINICAL STUDIES sections).
Geriatric Use—Healthy geriatric volunteers (≥65 years old) with normal renal function who received a single 400-mg dose of loracarbef had no significant differences in AUC or clearance when compared to healthy adult volunteers 20 to 40 years of age (see CLINICAL PHARMACOLOGY). Of 3541 adult patients in controlled clinical studies of loracarbef, 705 (19.9%) were 65 years of age or older. In these controlled clinical studies, when geriatric patients received the usual recommended adult doses, clinical efficacy and safety were comparable to results in non-geriatric patients.
Loracarbef is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because significant numbers of elderly patients have decreased renal function, care should be taken in dose selection and evaluation of renal function in this population is recommended (see DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
The nature of adverse reactions to loracarbef are similar to those observed with orally administered β-lactam antimicrobials. The majority of adverse reactions observed in clinical trials were of a mild and transient nature; 1.5% of patients discontinued therapy because of drug-related adverse reactions. No one reaction requiring discontinuation accounted for >0.03% of the total patient population; however, of those reactions resulting in discontinuation, gastrointestinal events (diarrhea and abdominal pain) and skin rashes predominated.
All Patients
The following adverse events, irrespective of relationship to drug, have been reported following the use of Lorabid in clinical trials. Incidence rates (combined for all dosing regimens and dosage forms) were less than 1% for the total patient population, except as otherwise noted:
Gastrointestinal: The most commonly observed adverse reactions were related to the gastrointestinal system. The incidence of gastrointestinal adverse reactions increased in patients treated with higher doses. Individual event rates included diarrhea, 4.1%; nausea, 1.9%; vomiting, 1.4%; abdominal pain, 1.4%; and anorexia.
Hypersensitivity: Hypersensitivity reactions including, skin rashes (1.2%), urticaria, pruritus, and erythema multiforme.
Central Nervous System: Headache (2.9%), somnolence, nervousness, insomnia, and dizziness.
Hemic and Lymphatic Systems: Transient thrombocytopenia, leukopenia, and eosinophilia.
Hepatic: Transient elevations in AST (SGOT), ALT (SGPT), and alkaline phosphatase.
Renal: Transient elevations in BUN and creatinine.
Cardiovascular System: Vasodilatation.
Genitourinary: Vaginitis (1.3%), vaginal moniliasis (1.1%).
As with other β-lactam antibiotics, the following potentially severe adverse experiences have been reported rarely with loracarbef in worldwide post-marketing surveillance: anaphylaxis, hepatic dysfunction including cholestasis (with or without jaundice), prolongation of the prothrombin time with clinical bleeding in patients taking anticoagulants, and Stevens-Johnson syndrome.
Pediatric Patients
The incidences of several adverse events, irrespective of relationship to drug, following treatment with Lorabid were significantly different in the pediatric population and the adult population as follows:
| Event | Pediatric | Adult |
| Diarrhea | 5.8% | 3.6% |
| Headache | 0.9% | 3.2% |
| Rhinitis | 6.3% | 1.6% |
| Nausea | 0.0% | 2.5% |
| Rash | 2.9% | 0.7% |
| Vomiting | 3.3% | 0.5% |
| Somnolence | 2.1% | 0.4% |
| Anorexia | 2.3% | 0.3% |
β-Lactam Antimicrobial Class Labeling:
The following adverse reactions and altered laboratory test results have been reported in patients treated with β-lactam antibiotics:
Adverse Reactions—Allergic reactions, aplastic anemia, hemolytic anemia, hemorrhage, agranulocytosis, toxic epidermal necrolysis, renal dysfunction, and toxic nephropathy. As with other β-lactam antibiotics, serum sickness-like reactions have been reported rarely with loracarbef.
Several β-lactam antibiotics have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy should occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Altered Laboratory Tests—Increased prothrombin time, positive direct Coombs' test, elevated LDH, pancytopenia, and neutropenia.
OVERDOSAGE
Signs and Symptoms—The toxic symptoms following an overdose of β-lactams may include nausea, vomiting, epigastric distress, and diarrhea.
Loracarbef is eliminated primarily by the kidneys. Forced diuresis, peritoneal dialysis, hemodialysis, or hemoperfusion have not been established as beneficial for an overdose of loracarbef. Hemodialysis has been shown to be effective in hastening the elimination of loracarbef from plasma in patients with chronic renal failure.
DOSAGE AND ADMINISTRATION
Lorabid is administered orally either at least 1 hour prior to eating or at least 2 hours after eating. The recommended dosages, durations of treatment, and applicable patient populations are described in the following chart:
| DOSAGE | Duration | |
| POPULATION/INFECTION | (mg) | (days) |
| ADULTS (13 years and older) | ||
| Lower Respiratory Tract | ||
| Secondary Bacterial Infection of Acute Bronchitis | 200–400 q12h | 7 |
| Acute Bacterial Exacerbation of Chronic Bronchitis | 400 q12h | 7 |
| Pneumonia | 400 q12h | 14 |
| Upper Respiratory Tract | ||
| Pharyngitis/Tonsillitis | 200 q12h | 10a |
| Sinusitis | 400 q12h | 10 |
| (SeeCLINICAL STUDIESand INDICATIONS AND USAGE for further information.) | ||
| Skin and Skin Structure | ||
| Uncomplicated Skin and Skin Structure Infections | 200 q12h | 7 |
| Urinary Tract | ||
| Uncomplicated cystitis | 200 q24h | 7 |
| (SeeCLINICAL STUDIESandINDICATIONS AND USAGE for further information.) | ||
| Uncomplicated pyelonephritis | 400 q12h | 14 |
| PEDIATRIC PATIENTS (6 months to 12 years) | ||
| Upper Respiratory Tract | ||
| Acute Otitis Mediab | 30 mg/kg/day in | 10 |
| divided doses q12h | ||
| (See CLINICAL STUDIESandINDICATIONS AND USAGE for further information.) | ||
| Acute maxillary sinusitis | 30 mg/kg/day in | 10 |
| divided doses q12h | ||
| (See CLINICAL STUDIESandINDICATIONS AND USAGE for further information.) | ||
| Pharyngitis/Tonsillitis | 15 mg/kg/day in | 10a |
| divided doses q12h | ||
| Skin and Skin Structure | ||
| Impetigo | 15 mg/kg/day in | 7 |
| divided doses q12h | ||
| a In the treatment of infections due to S. pyogenes, Lorabid should be administered for at least 10 days. | ||
|
b Otitis media should be treated with the suspension. Clinical studies of otitis media were conducted with the suspension formulation only. The suspension is more rapidly absorbed than the capsules, resulting in higher peak plasma concentrations when administered at the same dose. Therefore, the capsule should not be substituted for the suspension in the treatment of otitis media (see CLINICAL PHARMACOLOGY). |
||
| 100 mg/5 mL Suspension | 200 mg/5 mL Suspension | ||||
| Weight | Dose given twice daily | Dose given twice daily | |||
| lb | kg | mL | tsp | mL | tsp |
| 15 | 7 | 2.6 | 0.5 | — | — |
| 29 | 13 | 4.9 | 1.0 | 2.5 | 0.5 |
| 44 | 20 | 7.5 | 1.5 | 3.8 | 0.75 |
| 57 | 26 | 9.8 | 2.0 | 4.9 | 1.0 |
| 100 mg/5 mL Suspension | 200 mg/5 mL Suspension | ||||
| Weight | Dose given twice daily | Dose given twice daily | |||
| lb | kg | mL | tsp | mL | tsp |
| 15 | 7 | 5.2 | 1.0 | 2.6 | 0.5 |
| 29 | 13 | 9.8 | 2.0 | 4.9 | 1.0 |
| 44 | 20 | — | — | 7.5 | 1.5 |
| 57 | 26 | — | — | 9.8 | 2.0 |
Renal Impairment: Lorabid may be administered to patients with impaired renal function. The usual dose and schedule may be employed in patients with creatinine clearance levels of 50 mL/min or greater. Patients with creatinine clearance between 10 and 49 mL/min may be given half of the recommended dose at the usual dosage interval, or the normal recommended dose at twice the usual dosage interval. Patients with creatinine clearance levels less than 10 mL/min may be treated with the recommended dose given every 3 to 5 days; patients on hemodialysis should receive another dose following dialysis.
When only the serum creatinine is available, the following formula (based on sex, weight, and age of the patient) may be used to convert this value into creatinine clearance (CLcr, mL/min). The equation assumes the patient's renal function is stable.
| (weight in kg) x (140 - age) | |
| Males = | (72) x serum creatinine (mg/100 mL) |
| Females = | (0.85) x (above value) |
| Bottle Size | Reconstitution Direction |
| 100 mL | Invert the bottle and tap to loosen powder. Add 60 mL of water in 2 portions to the dry mixture in the bottle. |
| Shake well after each addition. |
After mixing, the suspension may be kept at room temperature, 15–30°C (59–86°F), for 14 days without significant loss of potency. Keep tightly closed. Discard unused portion after 14 days.
HOW SUPPLIED
Pulvules:
- 200 mg, (blue and gray) (15s) NDC 54868-2927-0.
- 400 mg, (blue and pink).
Keep tightly closed. Store at 25°C (77°F); excursion permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature]. Protect from heat.
CLINICAL STUDIES
ACUTE OTITIS MEDIA
Study 1
In a controlled clinical study of acute otitis media performed in the United States where significant rates of β-lactamase-producing organisms were found, loracarbef was compared to an oral antimicrobial agent that contained a specific β-lactamase inhibitor. In this study, using very strict evaluability criteria and microbiologic and clinical response criteria at the 10- to 16-day post therapy follow-up, the following presumptive bacterial eradication/clinical cure outcomes (ie, clinical success) and safety results were obtained:
| Efficacy: | ||
| % of Cases | ||
| Pathogen | With Pathogens | Outcome |
| (n=204) | ||
| S. pneumoniae | 42.6% | Loracarbef equivalent to control |
| H. influenzae | 30.4% | Loracarbef success rate 9% less than control |
| M. catarrhalis | 20.6% | Loracarbef success rate 19% less than control |
| S. pyogenes | 6.4% | Loracarbef equivalent to control |
| Overall | 100.0% | Loracarbef success rate 12% less than control |
Safety
The incidences of the following adverse events were clinically and statistically significantly higher in the control arm versus the loracarbef arm.
| *The majority of these involved the diaper area in young pediatric patients. | ||
| Event | Loracarbef | Control |
| Diarrhea | 15% | 26% |
| Rash* | 8% | 15% |
Study 2
In a controlled clinical study of acute otitis media performed in Europe, loracarbef was compared to amoxicillin. As expected in a European population, this study population had a lower incidence of β-lactamase-producing organisms than usually seen in Us.S. trials. In this study, using very strict evaluability criteria and microbiologic and clinical response criteria at the 10- to 16-day post therapy follow-up, the following presumptive bacterial eradication/clinical cure outcomes (ie, clinical success) were obtained:
| Efficacy: | ||
| % of Cases | ||
| Pathogen | With Pathogens | Outcome |
| (n=291) | ||
| S. pneumoniae | 51.5% | Loracarbef equivalent to amoxicillin |
| H. influenzae | 29.2% | Loracarbef success rate 14% greater than amoxicillin |
| M. catarrhalis | 15.8% | Loracarbef success rate 31% greater than amoxicillin |
| S. pyogenes | 3.4% | Loracarbef equivalent to amoxicillin |
| Overall | 100.0% | Loracarbef equivalent to amoxicillin |
ACUTE MAXILLARY SINUSITIS
In a controlled clinical study of acute maxillary sinusitis performed in Europe, loracarbef was compared to doxycycline. In this study there were 210 sinus-puncture evaluable patients. As expected in a European population, this study population had a lower incidence of β-lactamase-producing organisms than usually seen in U.S. trials. In this study, using very strict evaluability criteria and microbiologic and clinical response criteria at the 1- to 2-week post therapy follow-up, the following presumptive bacterial eradication/clinical cure outcomes (ie, clinical success) were obtained:
| Efficacy: | ||
| % of Cases | ||
| Pathogen | With Pathogens | Outcome |
| (n=210) | ||
| S. pneumoniae | 47.6% | Loracarbef equivalent to doxycycline |
| H. influenzae | 41.4% | Loracarbef equivalent to doxycycline |
| M. catarrhalis | 11.0% | Loracarbef equivalent to doxycycline |
| Overall | 100.0% | Loracarbef equivalent to doxycycline |
CYSTITIS
Study 1
In a controlled clinical study of cystitis performed in the United States, loracarbef was compared to cefaclor. In this study, using very strict evaluability criteria and microbiologic and clinical response criteria at the 5- to 9-day post therapy follow-up, the following bacterial eradication rates were obtained:
| Efficacy: | ||
| % of Cases | ||
| Pathogen | With Pathogens | Outcome |
| (n=186) | ||
| E. coli | 77.4% | Loracarbef eradication rate 4% greater than cefaclor (loracarbef eradication rate 80%) |
| Other major | 12.5% | Loracarbef equivalent to cefaclor |
| Enterobacteriaceae | (loracarbef eradication rate 61%) | |
| S. saprophyticus | 3.8% | Loracarbef equivalent to cefaclor |
Study 2
In a second controlled clinical study of cystitis, performed in Europe, loracarbef was compared to an oral quinolone. In this study, using very strict evaluability criteria and microbiologic and clinical response criteria at the 5- to 9-day post therapy follow-up, the following bacterial eradication rates were obtained: European Uncomplicated Cystitis Study Loracarbef vs Quinolone.
| Efficacy: | ||
| % of Cases | ||
| Pathogen | With Pathogens | Outcome |
| (n=189) | ||
| E. coli | 82.0% | Loracarbef eradication rate 7% greater than quinolone (loracarbef eradication rate 81%) |
| Other major | 10.1% | Loracarbef eradication rate 32% less |
| Enterobacteriaceae | than quinolone (loracarbef eradication rate 50%) | |
Pulvules® is a licensed registered trademark of Eli Lilly & Co.
REFERENCES
- National Committee for Clinical Laboratory Standards, M2- A4 performance standards for antimicrobial disk susceptibility tests. ed 4, Villanova, PA, April, 1990.
- National Committee for Clinical Laboratory Standards, M7-A2 methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. ed 2, Villanova, PA, April, 1990.
Rx Only.
Prescribing Information as of September 2002
Distributed by: Monarch Pharmaceuticals, Inc., Bristol, TN 37620
Manufactured by: Eli Lilly Italia, S.p.A., Sesto Fiorentino (Firenze), Italy
| LORABID
loracarbef capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Physicians Total Care, Inc. (194123980) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Physicians Total Care, Inc. | 194123980 | relabel(54868-2927) | |