DELOS - benzoyl peroxide lotion
Rochester Pharmaceuticals
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
PURPOSE
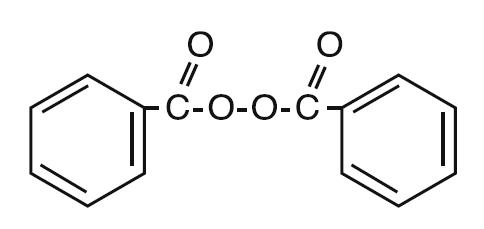
Delos™ (3.5% benzoyl peroxide) lotion is a topical preparation containing benzoyl peroxide, for use in the treatment of acne vulgaris, and patented Novasome encapsulation technology to help provide continuous hydration and moisturization to minimize the appearance of skin dryness. Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic agent. Benzoyl peroxide (C14H O4) is represented by the following chemical structure:
CLINICAL PHARMACOLOGY
The mechanism of action of benzoyl peroxide is not totally understood but its antibacterial activity against Propionibacterium acnes is thought to be a major mode of action, in addition, patients treated with benzoyl peroxide show a reduction in lipids and free fatty acids, and mild desquamation (drying and peeling activity) with simultaneous reduction in comedones and acne lesions. Little is known about the percutaneous penetration, metabolism, and excretion of benzoyl peroxide, although it has been shown that benzoyl peroxide absorbed by the skin is metabolized to benzoic acid and then excreted as benzoate in the urine. There is no evidence of systemic toxicity caused by benzoyl peroxide in humans.
INGREDIENTS
Delos™ (3.5% benzoyl peroxide) lotion contains benzoyl peroxide USP 3.5% as the active ingredient.
INACTIVE INGREDIENTS
Allantoin, Aloe Barbadensis Leaf Juice, Butylene Glycol, Caprylic/Capric Triglyceride, Cetearyl Alcohol, Cetearyl Glucoside, Cetearyl Olivate, Cetyl Alcohol, Dimethicone, Edetate Disodium, Ethylene/Acrylic Acid Copolymer, Glycerin, Glycine Soya (Soybean) Sterols, Methylparaben, Phenoxyethanol, Propylparaben, Purified Water, Sorbitan Olivate, Stearyl Alcohol, Tocopheryl Acetate, and Xanthan Gum.
INDICATIONS & USAGE
Delos™ (3.5% benzoyl peroxide) lotion is indicated for the topical treatment of acne vulgaris.
CONTRAINDICATIONS
These preparations are contraindicated in patients with a history of hypersensitivity to any of their components.
PRECAUTIONS
General
For external use only. If severe irritation develops, discontinue use and institute appropriate therapy. After reaction clears, treatment may often be resumed with less frequent application. These preparations should not be used in or near the eyes or on mucous membranes.
Information for Patients
Avoid contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. Contact with any colored material (including hair and fabric) may result in bleaching or discoloration. If excessive irritation develops, discontinue use and consult your physician.
Carcinogenesis, Mutagenesis, Impairment
Data from several studies employing a strain of mice that are highly susceptible to developing cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of these findings to humans is unknown. Benzoyl peroxide has not been found to be mutagenic (Ames Test) and there are no published data indicating it impairs fertility.
Pregnancy: Teratogenic Effects: Pregnancy Category C
Animal reproduction studies have not been conducted with benzoyl peroxide. It is not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can effect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed. There are no available data on the effect of benzoyl peroxide on the later growth, development and functional maturation of the unborn child.
ADVERSE REACTIONS
Allergic contact dermatitis and dryness have been reported with topical benzoyl peroxide therapy.
OVERDOSAGE
If excessive scaling, erythema or edema occurs, the use of these preparations should be discontinued. To hasten resolution of the adverse effects, cool compresses may be used. After symptoms and signs subside, a reduced dosage schedule may be cautiously tried if the reaction is judged to be due to excessive use and not allergenicity.
DOSAGE & ADMINISTRATION
Apply Delos™ (3.5% benzoyl peroxide) lotion once or twice daily to cover affected areas, or as directed by your physician. Use after washing with a mild cleanser and water.
HOW SUPPLIED
Delos™ (3.5% benzoyl peroxide) lotion is available in 45 g tube (NDC 49908-121-45).
Store at controlled room temperature: 15° - 30° C (59° - 86° F).
Manufactured for:
Rochester Pharmaceuticals
Rochester, NY 14625
Toll Free: 1-866-458-1772
www.RoshesterPharm.com
www.DelosLotion.com
August 2010
PACKAGE LABEL

NDC 49908-121-45
For dermatologic use only - Not for ophthalmic use
Rx only
delos™ with Novasome™ Technology
(3.5% benzoyl peroxide) lotion
1.5 oz (45 g)
Each gram contains - Active: benzoyl peroxide 3.5%. Inactive: Allantoin, Aloe Barbadensis Leaf Juice, Butylene Glycol, Caprylic/Capric Triglyceride, Cetearyl Alcohol, Cetearyl Glucoside, Cetearyl Olivate, Cetyl Alcohol, Dimethicone, Edetate Disodium, Ethylene/Acrylic Acid Copolymer, Glycerin, Glycine Soya (Soybean) Sterols, Methylparaben, Phenoxyethanol, Propylparaben, Purified Water, Sorbitan Olivate, Stearyl Alcohol, Tocopheryl Acetate, and Xanthan Gum.
Usual dosage: Apply a thin layer to entire affected areas after washing. Use morning and evening or as directed by physician.
Avoid application close to the eyes. See package insert for full prescribing information.
Important: Do not use if seal has been punctured or is not visible.
Store at 15°-30°C (59°-86°F)
See crimp for lot no. and expiration date.
Distributed by: Rochester Pharmaceuticals
Rochester, NY 14625 • www.RochesterPharm.com
Rochester™ Pharmaceuticals

NDC 49908-121-45
For dermatologic use only - Not for ophthalmic use
Rx only
delos™ with Novasome™ Technology
(4.5% benzoyl peroxide) lotion
1.5 oz (45 g)
Each gram contains - Active: benzoyl peroxide 3.5%. Inactive: Allantoin, Aloe Barbadensis Leaf Juice, Butylene Glycol, Caprylic/Capric Triglyceride, Cetearyl Alcohol, Cetearyl Glucoside, Cetearyl Olivate, Cetyl Alcohol, Dimethicone, Edetate Disodium, Ethylene/Acrylic Acid Copolymer, Glycerin, Glycine Soya (Soybean) Sterols, Methylparaben, Phenoxyethanol, Propylparaben, Purified Water, Sorbitan Olivate, Stearyl Alcohol, Tocopheryl Acetate, and Xanthan Gum.
Usual dosage: Apply a thin layer to entire affected areas after washing. Use morning and evening or as directed by physician.
Avoid application close to the eyes. See package insert for full prescribing information.
Important: Do not use if seal has been punctured or is not visible.
Store at 15°-30°C (59°-86°F)
See crimp for lot no. and expiration date.
Distributed by: Rochester Pharmaceuticals
Rochester, NY 14625 • www.RochesterPharm.com
Rochester™ Pharmaceuticals
| DELOS
benzoyl peroxide lotion |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Rochester Pharmaceuticals (069874500) |