IMMEDIATE RELEASE MUCUS RELIEF- guaifenesin tablet
Safeway
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Safeway 44-532
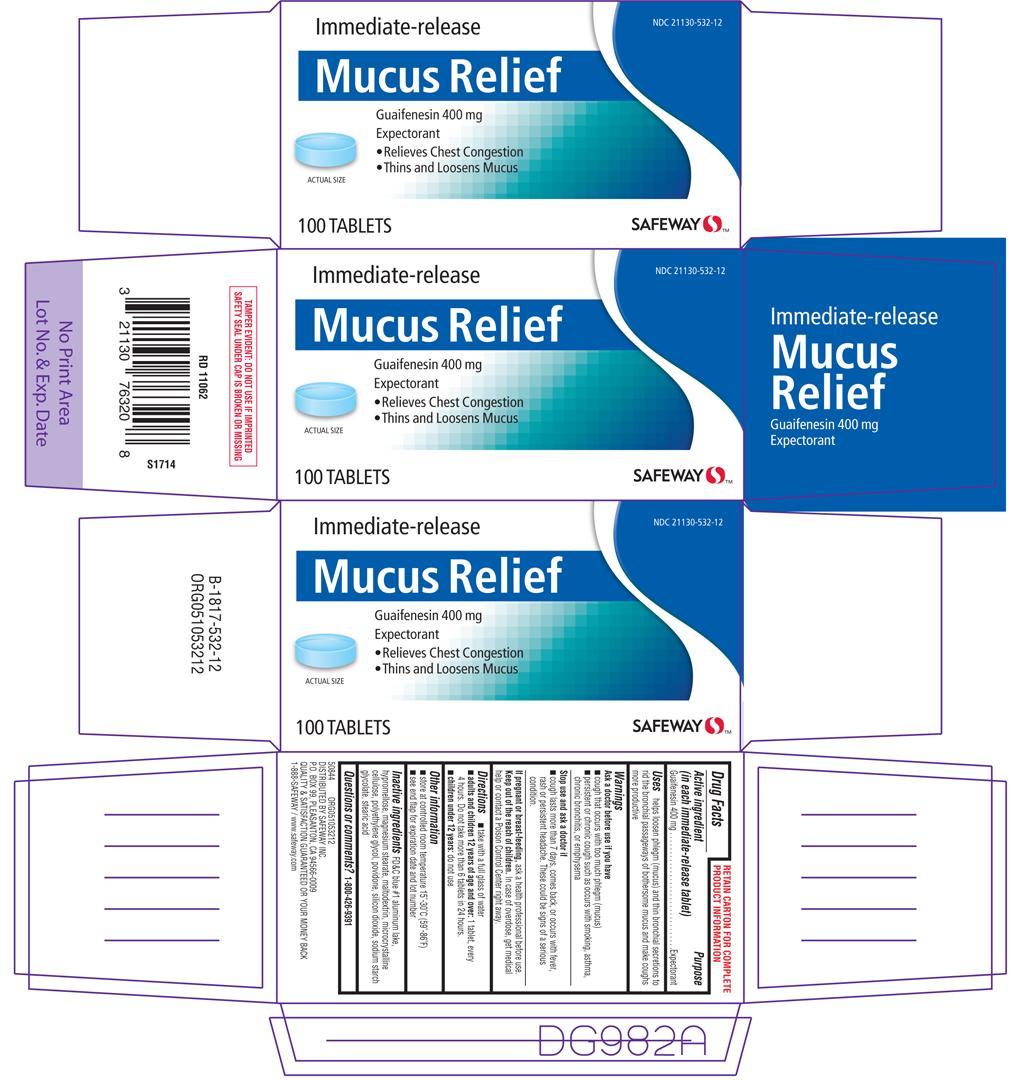
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersom mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough that occurs with too much phlegm (mucus)
Directions
- take with a full glass of water
- adults and children 12 years of age and over: 1 tablet, every 4 hours. Do not take more than 6 tablets in 24 hours.
- children under 12 years: do not use
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
FD&C blue #1 alluminum lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, silicon dioxide, sodium starch glycolate, stearic acid
Principal Display Panel
NDC 21130-555-01
Immediate-release
Mucus Relief
Guaifenesin 400 mg
Expectorant
•Relieves Chest Congestion
•Thins and Loosens Mucus
30 TABLETS
SAFEWAY™
50844 REV0812D53201
DISTRIBUTED BY SAFEWAY INC., P.O. BOX 99, PLEASANTON, CA 94566-009
QUALITY & SATISFACTION GUARANTEED OR YOUR MONEY BACK
1-888-SAFEWAY / www.safeway.com
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Safeway 44-532
| IMMEDIATE RELEASE MUCUS RELIEF
guaifenesin tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Safeway (009137209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(21130-555) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(21130-555) | |